Cross-Linking in Polylactic Acid: Strategic Modulation of Crystallization Behavior and Degradation Kinetics for Advanced Applications
This review provides a comprehensive analysis of how cross-linking strategies fundamentally alter the crystallization dynamics and degradation profiles of poly(lactic acid) (PLA) and its copolymers.
Cross-Linking in Polylactic Acid: Strategic Modulation of Crystallization Behavior and Degradation Kinetics for Advanced Applications
Abstract
This review provides a comprehensive analysis of how cross-linking strategies fundamentally alter the crystallization dynamics and degradation profiles of poly(lactic acid) (PLA) and its copolymers. We systematically examine the foundational principles governing cross-link formation in PLA systems, explore advanced methodological approaches for their implementation, address critical troubleshooting and optimization challenges, and present rigorous validation through comparative performance analysis. By synthesizing recent scientific advances, this article establishes clear structure-property relationships crucial for researchers and drug development professionals seeking to engineer PLA-based materials with precisely tailored degradation rates and crystalline morphologies for biomedical devices, controlled drug delivery systems, and sustainable packaging applications.
Fundamental Mechanisms: How Cross-Linking Chemically Alters PLA Crystallization and Degradation Pathways
Poly(lactic acid) (PLA) is a leading biodegradable and bio-based aliphatic polyester, derived from renewable resources like corn and sugarcane, positioning it as a sustainable alternative to conventional petroleum-based plastics [1]. Its excellent biocompatibility and comparable mechanical properties to many engineering plastics have fueled its adoption in fields ranging from biomedical implants to food packaging [2] [1]. However, the widespread application of PLA is often constrained by inherent limitations, such as insufficient melt strength for foaming, slow crystallization rate, and relatively low heat resistance [3] [4] [5].
To overcome these challenges, strategic modifications of PLA’s polymer architecture are essential. Among these, the formation of cross-linked networks has emerged as a powerful technique. Cross-linking can significantly enhance PLA's thermal stability, mechanical properties, and melt strength, which is particularly crucial for producing high-performance foams and durable goods [3] [1]. This review provides a comparative analysis of two primary strategies for forming cross-links in PLA systems: in-situ cross-linking during processing and reactive compatibilization in polymer blends. The discussion is framed within the context of a broader thesis, investigating how these cross-link formation methods distinctly influence two critical aspects of PLA performance: its crystallization behavior and its hydrolytic degradation profile. Understanding these structure-property relationships is vital for researchers and scientists to tailor PLA materials for specific applications, whether the goal is to delay degradation for long-term implants or accelerate it for disposable packaging.
In-situ Cross-Linking Strategies
In-situ cross-linking refers to the process of forming covalent bonds between polymer chains during the material's processing, such as extrusion or hot-pressing. This method is highly valued for its ability to enhance the melt strength and thermo-mechanical properties of PLA without requiring pre-modification of the base resin.
Chemical Cross-Linking via Peroxide Initiators
A prominent in-situ approach involves the use of peroxide initiators and co-agents. In one study, a cross-linked network was successfully created in a PLA/poly(4-hydroxybutyrate) (P4HB) blend using bis(tert-butylperoxy isopropyl) benzene (BIBP) as the peroxide initiator and triallyl isocyanurate (TAIC) as the co-agent [3]. The mechanism involves BIBP decomposing under heat to generate free radicals, which then abstract hydrogen from the polymer backbone. The TAIC, with its multiple allyl groups, readily reacts with these polymer radicals, forming a cross-linked network that bridges PLA and P4HB chains. This process not only increases the melt strength of the blend but also compatibilizes the phases, reducing interfacial stress concentrations [3]. The resulting cross-linked foams exhibited a closed-cell structure with a high volume expansion ratio (VER), contributing to excellent impact resistance and thermal insulation properties [3].
Table 1: Key Reagents for Chemical Cross-Linking of PLA
| Reagent Name | Function | Mechanism & Role |
|---|---|---|
| Bis(tert-butylperoxy isopropyl) benzene (BIBP) | Peroxide Initiator | Decomposes thermally to generate free radicals that initiate cross-linking by abstracting hydrogen from polymer chains. |
| Triallyl Isocyanurate (TAIC) | Cross-linking Co-agent | Multi-functional monomer whose allyl groups form bridges between polymer radicals, creating a 3D network. |
| Trimethylolpropane triacrylate (TMPTA) | Cross-linking Co-agent | Acrylate-functionalized molecule that reacts with radicals to link multiple polymer chains. |
Cross-Linking via High-Energy Irradiation
An alternative physical method for in-situ cross-linking employs high-energy irradiation, such as electron beam (E-beam) or gamma rays. When PLA is exposed to ionizing radiation, radicals are generated directly on its polymer chains, which can subsequently recombine to form cross-links [1]. However, PLA has a tendency to undergo chain scission under irradiation. To promote cross-linking over degradation, polyfunctional monomers like TAIC, trimethallyl isocyanurate (TMAIC), or trimethylolpropane triacrylate (TMPTA) are often added [1]. These monomers possess multiple reactive sites that enhance the efficiency of network formation. The cross-linked PLA materials produced via irradiation display improved heat stability and can become rubbery and stable at temperatures even above their melting point (( T_m )) [1].
The following diagram illustrates the decision-making workflow for selecting and implementing an in-situ cross-linking strategy for PLA, incorporating both chemical and irradiation methods.
Reactive Compatibilization in Blends
Reactive compatibilization is a powerful strategy for creating cross-like structures at the interface of polymer blends, particularly for immiscible pairs like PLA and polyolefins. This method improves adhesion between phases and stabilizes the blend morphology, leading to enhanced mechanical properties.
Mechanism and Reagents
The core principle involves introducing a reactive component that can chemically interact with both phases of the blend during melt processing. For instance, in PLA/PE blends, telechelic hydroxyl-functional PE can be synthesized to react with the end groups of PLA chains [6]. The efficiency of this reaction can be dramatically accelerated by employing catalysts that localize at the interface, such as stannous octoate, leading to a finer dispersion of the dispersed phase and improved interfacial adhesion [6]. Similarly, in PLA/PP blends, a multifunctional agent like trimethylolpropane tri-acrylate (TMPTA) under in-situ UV irradiation can form PLA-TMPTA-PP copolymers, which act as compatibilizers, reducing the size of the dispersed PP domains and improving the overall blend compatibility [4].
Table 2: Key Reagents for Reactive Compatibilization of PLA Blends
| Reagent Name | Function | Application & Mechanism |
|---|---|---|
| Telechelic hydroxyl-functional PE | Reactive Polymer | End-functionalized polymer that reacts with PLA chain ends, forming a block copolymer at the interface of PLA/PE blends. |
| Trimethylolpropane tri-acrylate (TMPTA) | Multifunctional Agent | Forms copolymers with PLA and PP under UV irradiation, compatibilizing the blend interface. |
| Stannous Octoate | Catalyst | Localizes at the polymer-polymer interface and accelerates transesterification or coupling reactions. |
Comparative Effects on Crystallization
The introduction of cross-links and compatibilized structures has a profound and complex impact on the crystallization behavior of PLA, which is a critical factor determining its final mechanical and thermal properties.
Nucleation and Crystal Growth
Cross-linked networks can influence crystallization in two opposing ways. On one hand, they can restrict the mobility of polymer chains, thereby impeding the growth of large crystals and potentially reducing the overall degree of crystallinity [7]. On the other hand, the interfaces in compatibilized blends or the cross-linked network itself can act as nucleation sites. Research on reactively compatibilized PLA/PP blends showed that the compatibilized interface assisted in the nucleation of PLA, leading to an increased spherulite density compared to neat PLA [4]. The cross-linking strategy in PLA/P4HB blends was also designed to increase the number of cell nucleation sites during foaming, which is indirectly linked to the crystallization behavior of the polymer matrix [3].
Crystal Form and Stability
PLA can crystallize in different forms, with the α-form being the most thermodynamically stable and the α′-form being a metastable disordered form that develops at lower crystallization temperatures [2]. The crystal form has a significant effect on material properties; α-form crystals generally provide a higher modulus and better barrier properties but can lead to brittleness, whereas α′-form structures are associated with higher tensile ductility [2]. The presence of a cross-linked network can alter the crystallization kinetics and the conditions under which these different forms develop, thereby providing a means to control the final material properties. For example, a higher cross-link density would be expected to favor the formation of the less ordered α′-form due to restricted chain mobility.
Table 3: Comparative Data: Cross-Link Effects on Crystallization and Properties
| System & Strategy | Effect on Crystallization | Resultant Key Property Changes |
|---|---|---|
| PLA/P4HB + In-situ Cross-link [3] | Increased melt strength; increased number of nucleation sites. | Formation of closed-cell foams with high volume expansion ratio (VER); excellent impact resistance and thermal insulation. |
| PLA/PP + Reactive Compatibilization [4] | Interface-assisted nucleation; increased PLA spherulite density. | Improved dispersion (smaller PP domains); enhanced compatibility and mechanical properties of the blend. |
| Cross-linked PLLA Networks [7] | Restricted chain mobility; reduced crystallinity (can be made amorphous with low ( M_c )). | Enhanced tensile strength; retained form stability during degradation. |
| General α-form vs. α′-form PLA [5] [2] | α-form: higher crystallinity, more ordered. α′-form: lower crystallinity, disordered. | α-form: Higher modulus, better heat resistance, better barrier properties. α′-form: Higher ductility. |
Comparative Effects on Hydrolytic Degradation
The degradation profile of PLA is a paramount consideration, especially for biomedical and environmental applications. Cross-linking fundamentally alters the hydrolysis mechanism and kinetics.
Mechanism: Surface Erosion vs. Bulk Erosion
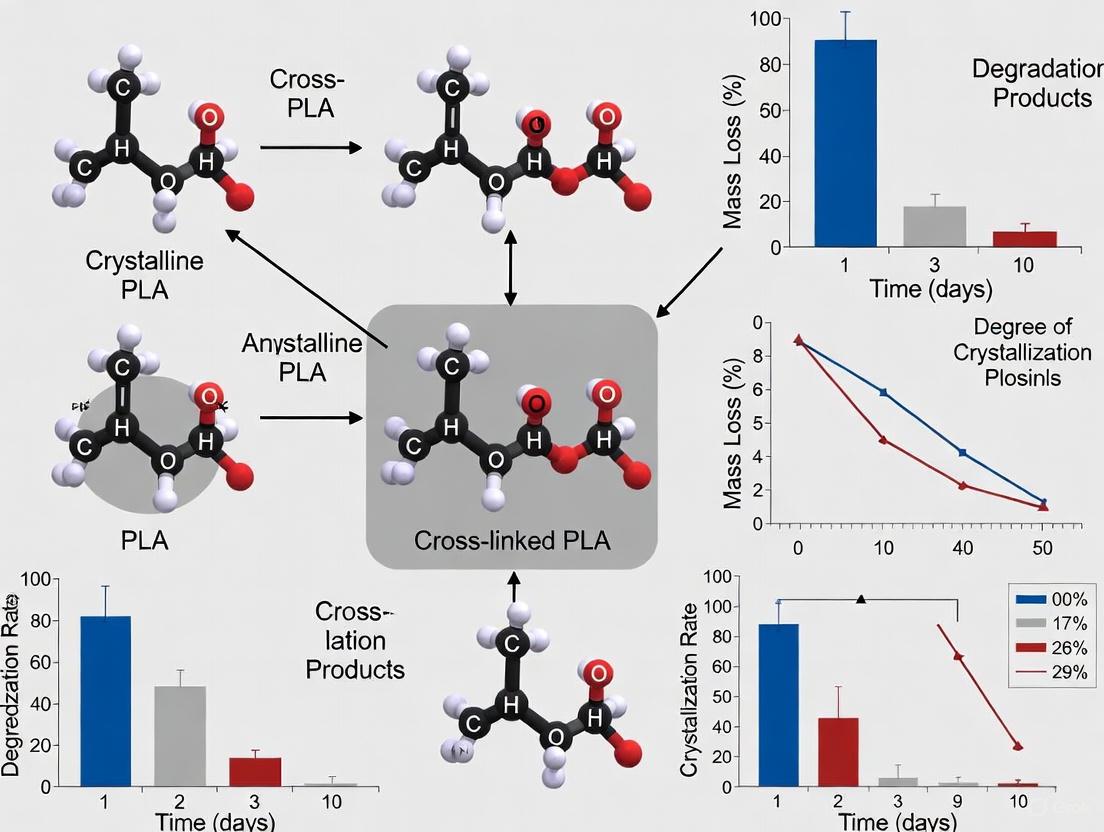
Linear, semi-crystalline PLA typically degrades via bulk erosion, where water penetrates the entire specimen, causing random chain scission throughout the material. This leads to a gradual loss of mechanical properties while the mass remains largely unchanged until the material finally fragments [7]. In contrast, cross-linked PLA networks tend to degrade via surface erosion. In this mechanism, hydrolysis occurs primarily at the surface of the material, leading to a linear mass loss over time while the bulk of the material maintains its mechanical integrity and form stability for a longer period [7]. This phenomenon is because the dense network structure hinders the penetration of water into the bulk.
The Role of Cross-link Density
The density of the cross-linked network, often characterized by the average molecular weight between cross-links (( Mc )), is a critical factor controlling the degradation rate. Studies on PLLA-based networks have shown that a lower ( Mc ) (higher cross-link density) results in a slower degradation rate and confines the degradation to a thinner layer at the surface [7]. For example, a network with an ( Mc ) of 1400 g/mol showed degradation confined to the outer surface, whereas a network with an ( Mc ) of 3500 g/mol exhibited degradation up to a 400 μm layer from the surface [7]. Furthermore, the crystalline regions that form during the degradation process, as the chains become more mobile, can further slow down the degradation, as they are more resistant to hydrolysis than amorphous regions [2] [8].
The diagram below summarizes the divergent degradation pathways for linear versus cross-linked PLA and the key factors influencing the process.
The Scientist's Toolkit: Essential Reagents & Methodologies
This section provides a consolidated reference for the key reagents and experimental protocols central to researching cross-link formation in PLA.
Key Research Reagent Solutions
Table 4: Essential Reagents for PLA Cross-Linking Research
| Category | Reagent | Primary Function in PLA Research |
|---|---|---|
| Peroxide Initiators | Bis(tert-butylperoxy isopropyl) benzene (BIBP) | Generates free radicals to initiate cross-linking reactions during thermal processing. |
| Cross-linking Co-agents | Triallyl Isocyanurate (TAIC), Trimethylolpropane triacrylate (TMPTA) | Multi-functional monomers that bridge polymer radicals to form a three-dimensional network. |
| Catalysts | Stannous Octoate (Sn(Oct)â‚‚) | Catalyzes transesterification and coupling reactions, especially effective at polymer interfaces. |
| Reactive Polymers | Telechelic hydroxyl-functional PE | Acts as a macromolecular compatibilizer by reacting with PLA end-groups in blends. |
| TK216 | TK216 is a small molecule inhibitor for research, targeting ETS transcription factors and microtubules. It is for research use only (RUO). Not for human consumption. | |
| INDY | INDY, MF:C12H13NO2S, MW:235.30 g/mol | Chemical Reagent |
Detailed Experimental Protocol: In-situ Cross-Linking and Foaming
The following methodology, adapted from a cited study, outlines a standard procedure for creating and evaluating in-situ cross-linked PLA foams [3].
1. Materials Preparation:
- Polymer Resins: Dry PLA (e.g., LX175) and P4HB pellets in a vacuum oven at 80°C for 24 hours to prevent hydrolysis during processing.
- Cross-linking Additives: Weigh bis(tert-butylperoxy isopropyl) benzene (BIBP) and triallyl isocyanurate (TAIC) accurately. Typical concentrations are in the range of 0.5-1.5 parts per hundred resin (phr) for BIBP and 1-3 phr for TAIC.
2. Melt Blending and Cross-Linking:
- Utilize an internal mixer (e.g., Haake Rheomix) or a twin-screw extruder.
- Set the processing temperature to a range that melts the polymers but is below the rapid decomposition temperature of the peroxide (e.g., 170-190°C).
- Introduce the dried PLA and P4HB pellets and allow them to melt and mix.
- Add the TAIC co-agent and BIBP peroxide initiator to the melt.
- Mix for a specific time (e.g., 5-10 minutes) to ensure homogeneous dispersion and allow the cross-linking reaction to proceed.
3. Foaming Process with Supercritical COâ‚‚:
- The blended and cross-linked material is compression-molded into sheets.
- The sheets are placed in a high-pressure vessel, which is then heated to the foaming temperature (e.g., 120-160°C).
- Introduce supercritical COâ‚‚ (sc-COâ‚‚) as the physical blowing agent at a specified pressure (e.g., 10-20 MPa) and allow sufficient time for saturation.
- Release the pressure rapidly to induce thermodynamic instability and cell nucleation, resulting in a foam structure.
4. Characterization and Analysis:
- Cell Structure: Analyze the foam morphology (cell size, density, open/closed cell content) using scanning electron microscopy (SEM).
- Gel Content: Determine the extent of cross-linking by Soxhlet extraction with an appropriate solvent (e.g., CHCl₃) to measure the insoluble gel fraction.
- Thermal Properties: Use Differential Scanning Calorimetry (DSC) to analyze thermal transitions (Tg, Tc, Tm) and degree of crystallinity.
- Mechanical Performance: Perform impact resistance tests (e.g., Izod or Charpy) and compression tests to evaluate mechanical improvements.
- Melt Strength: Use a rheometer to measure the melt strength and viscoelastic properties of the cross-linked blend.
The strategic formation of cross-links in PLA, whether through in-situ techniques or reactive compatibilization, offers a powerful toolbox for enhancing its material properties. In-situ cross-linking with peroxides and co-agents is highly effective for improving melt strength and creating high-performance, closed-cell foams with excellent thermal insulation and impact resistance. Reactive compatibilization, on the other hand, is indispensable for creating high-performance PLA blends with otherwise immiscible polymers, leading to stabilized morphologies and enhanced mechanical properties.
The choice of strategy has profound and predictable consequences for both the crystallization behavior and the hydrolytic degradation of PLA. Cross-linking can tailor crystallization by providing nucleation sites while potentially limiting crystal growth, influencing toughness and heat resistance. More distinctly, it shifts the degradation mechanism from bulk to surface erosion, providing a means to control the lifetime and maintain the structural integrity of PLA products in use. For researchers and product developers, the decision between these strategies must be guided by the application's specific requirements: seeking enhanced thermo-mechanical performance and processability, or precisely managing the degradation profile for biomedical or specific environmental end-of-life scenarios.
Poly(lactic acid) (PLA) is a leading biobased and biodegradable polymer with significant potential to replace petroleum-based plastics in applications ranging from medical devices to packaging [9]. However, its widespread adoption is limited by inherent shortcomings, including slow crystallization kinetics, brittleness, and low heat deflection temperature [9] [10]. Crosslinking has emerged as a promising strategy to enhance PLA's mechanical and thermal properties, creating a network structure that significantly influences crystallization behavior [1]. Understanding how crosslinking affects nucleation, crystal growth rates, and ultimate crystallinity is essential for tailoring material properties to specific applications.
The crystallization behavior of semi-crystalline polymers like PLA directly governs critical performance characteristics, including tensile strength, modulus, impact resistance, and thermal stability [5] [9]. For cross-linked PLA, the network structure imposes topological constraints on polymer chains, fundamentally altering their ability to rearrange into ordered crystalline structures. This creates a complex interplay between crosslink density, crystallization kinetics, and final material properties that researchers must navigate to optimize PLA for demanding applications where current performance is insufficient.
Crosslinking Methodologies for PLA
High-Energy Radiation Crosslinking
Crosslinking of PLA using high-energy radiation, particularly electron beam and gamma irradiation, represents a prominent physical modification method [1]. Unlike chemical crosslinking, this approach can be performed on pristine PLA without prerequisite functionalization, as radicals are generated directly on polymer chains upon irradiation. However, PLA predominantly undergoes chain scission rather than crosslinking when irradiated alone, leading to molecular weight reduction and property deterioration [1]. To overcome this limitation, polyfunctional monomers are incorporated as crosslinking co-agents.
Table 1: Crosslinking Co-Agents for Radiation-Induced PLA Crosslinking
| Co-Agent | Chemical Type | Optimal Concentration | Key Findings | Reference |
|---|---|---|---|---|
| Triallyl isocyanurate (TAIC) | Cyanurate derivative | ~3% | Most optimal conditions at 30-50 kGy dose; improved heat stability and mechanical properties | [1] |
| Trimethylolpropane trimethacrylate (TMPTMA) | Acrylate ester | 1-3% | Enhanced crosslinking density; increased gel content | [1] |
| 1,6-Hexanediol diacrylate (HDDA) | Di-functional acrylate | 1-3% | Improved network formation; retarded degradation | [1] |
The crosslinking process with these co-agents typically employs radiation doses between 30-50 kGy, with higher doses promoting increased crosslink density but risking predominant chain scission [1]. The resulting crosslinked PLA exhibits substantially improved heat stability and mechanical properties, particularly at elevated temperatures, where the material transitions to a rubbery state rather than melting [1].
Stereocomplex Crosslinking
An alternative physical crosslinking approach utilizes PLA stereocomplexation between poly(L-lactide) (PLLA) and poly(D-lactide) (PDLA) enantiomers [11]. This method creates a physical network through stereocomplex crystallites that act as crosslinking points, offering superior thermal stability compared to homocrystallized PLA. The stereocomplex formation requires remarkably short sequence lengths of only approximately 7 repeating units, making it particularly suitable for multiblock copolymers where longer sequences for homocrystallization may be sterically hindered [11].
In sophisticated material designs, researchers have developed multiblock copolymers such as PCL-PLLA and PGACL-PDLA, where one segment provides structural support while the other enables stereocomplex crosslinking [11]. This approach allows precise control over degradation profiles and mechanical property evolution during use, making it particularly valuable for biomedical applications like tissue fixation devices that require increased flexibility during the healing process [11].
Comparative Crystallization Kinetics: Cross-Linked vs. Modified PLA
Experimental Methodology for Crystallization Analysis
The investigation of crystallization kinetics in PLA systems relies on standardized experimental protocols that enable direct comparison between different modifications. Differential scanning calorimetry (DSC) serves as the primary analytical technique, with both isothermal and non-isothermal methods providing critical kinetic parameters [12] [13] [14].
Isothermal Crystallization Protocol: Samples are first heated from room temperature to 195°C at 30°C/min and held for 1 minute to erase thermal history [14] [15]. They are then rapidly cooled (80°C/min) to predetermined isothermal crystallization temperatures (typically between 80-140°C) and held until crystallization is complete, as indicated by the return of the exotherm to baseline [13] [14]. This protocol enables determination of crystallization half-time (tâ‚/â‚‚) and Avrami kinetic parameters.
Non-Isothermal Crystallization Protocol: Samples undergo heating from room temperature to 200°C at 10°C/min to determine glass transition temperature (Tg), cold crystallization temperature (Tcc), and melting temperature (Tm) [14]. Cooling rate variations provide insights into crystallization rate dependence on thermal history, particularly relevant for processing techniques like injection molding.
Complementary techniques include polarized optical microscopy (POM) for spherulitic morphology observation [5] [14], X-ray diffraction (XRD) for crystalline structure identification [12] [13], and wide-angle X-ray scattering (WAXS) for crystal form determination [5] [11].
Quantitative Crystallization Kinetics Data
Table 2: Comparative Crystallization Kinetics of PLA Systems
| PLA System | Modification Type | Half-Crystallization Time (tâ‚/â‚‚) | Crystallization Temperature (Tᶜ) | Maximum Crystallinity (%) | Reference |
|---|---|---|---|---|---|
| Neat PLA (3100HP) | Unmodified | >5 min (non-isothermal) | ~100°C (cooling) | 25-30% | [10] |
| PLA + 1% EBS | Nucleating Agent | Lowest at 110°C (isothermal) | Increased | ~40% | [13] |
| PLA + 1% Orotic Acid | Nucleating Agent | <5 min (from 80 min for neat PLA) | Reduced from 90°C to 70°C | Significantly increased | [13] |
| PLA + 15% Tributyrin | Plasticized | Decreased | Reduced | Enhanced | [12] [14] |
| PLA + 15% USOP | Bio-based Plasticizer | Decreased | Reduced | Enhanced | [12] [14] |
| Cross-linked PLA (with TAIC) | Cross-linked | Increased (constrained chains) | Unreported | Limited (~20% gel content) | [1] |
The data reveal that cross-linked PLA systems typically exhibit constrained crystallization kinetics due to restricted chain mobility, resulting in longer half-times and potentially lower ultimate crystallinity compared to nucleated or plasticized systems [1]. In contrast, nucleating agents like EBS and orotic acid dramatically accelerate crystallization, reducing half-times from minutes to seconds under optimal conditions [13]. Bio-based plasticizers such as used sunflower oil (USOP) also enhance crystallization rates by improving chain mobility, though less profoundly than specialized nucleating agents [12] [14].
Research Reagents and Materials Toolkit
Table 3: Essential Research Reagents for PLA Crystallization Studies
| Reagent/Material | Function | Application Context | Key Characteristics |
|---|---|---|---|
| Triallyl isocyanurate (TAIC) | Crosslinking co-agent | Radiation crosslinking | Polyfunctional; enhances crosslinking efficiency under e-beam/Gamma |
| PLLA/PDLA enantiomers | Stereocomplex formation | Physical crosslinking | Forms stereocomplex crystallites with higher Tm (~230°C) |
| Zinc PhenylPhosphonate | Nucleating agent | Crystallization enhancement | Most effective nucleating agent; significantly reduces tâ‚/â‚‚ |
| Orotic Acid (OA) | Organic nucleator | Crystallization acceleration | Reduces crystallization time from 80 min to <5 min at 1% loading |
| Ethylene Bis-Stearamide (EBS) | Nucleating agent | Crystallization promotion | At 1%, produces fastest crystallization at 110°C |
| Used Sunflower Oil (USOP) | Bio-based plasticizer | Flexibility & crystallization modifier | Increases spherulite size; reduces crystallization activation energy |
| Tributyrin (TB) | Conventional plasticizer | Flexibility & crystallization modifier | Reduces Tg; enhances crystallization rate more than USOP |
| AP521 | AP521, CAS:151227-08-6, MF:C20H19ClN2O3S, MW:402.9 g/mol | Chemical Reagent | Bench Chemicals |
| Xl-999 | XL999 | Bench Chemicals |
This toolkit provides researchers with essential materials for designing experiments to investigate and manipulate crystallization behavior in PLA systems. The selection of specific reagents depends on the target properties and modification strategy, whether aiming for enhanced crystallization rates (nucleating agents), improved flexibility (plasticizers), or network formation (crosslinking agents).
Interrelationship Between Modification Approaches
The following diagram illustrates the conceptual relationships between different PLA modification strategies and their subsequent effects on crystallization behavior and final material properties:
This conceptual framework reveals that crosslinking, nucleation, and plasticization exert distinct influences on the crystallization process. While nucleating agents and plasticizers generally promote crystallization kinetics through increased nucleation sites and enhanced chain mobility respectively, crosslinking typically restricts chain mobility and thus slows crystallization [13] [1] [14]. The optimal modification strategy depends critically on the target application and required balance between properties such as heat resistance, mechanical strength, and degradation behavior.
Implications for Material Performance and Applications
The crystallization behavior of cross-linked PLA directly governs its thermomechanical properties and practical applicability. For medical devices such as tissue fixation systems, the evolution of mechanical properties during degradation is particularly critical. Research demonstrates that properly designed cross-linked PLA systems can exhibit increasing flexibility during degradation—a desirable trait for supporting healing tissues [11]. This behavior contrasts with conventional PLA, which typically becomes more brittle as degradation proceeds.
In applications requiring enhanced heat resistance, such as food containers or electronic housings, achieving high crystallinity is essential for raising the heat deflection temperature (HDT). Studies show that highly crystallized PLA components can achieve HDT values approaching 90°C, making them suitable for higher-temperature applications [10]. However, crosslinked systems face inherent challenges in achieving high crystallinity degrees due to restricted chain mobility, potentially limiting their HDT enhancement compared to optimally nucleated systems.
The selection of modification approach ultimately depends on application requirements. Crosslinking excels where dimensional stability at elevated temperatures, controlled degradation profiles, or shape memory properties are prioritized. Nucleation strategies prove superior when maximum crystallinity and crystallization rate are essential for manufacturing efficiency and ultimate properties. Emerging hybrid approaches that combine multiple modification strategies offer promising avenues for overcoming individual limitations and achieving optimized property profiles for demanding applications.
Hydrolytic degradation is a fundamental process that governs the lifetime and performance of poly(lactic acid) (PLA) in applications ranging from packaging to biomedicine. For researchers and drug development professionals, understanding the precise mechanisms of ester bond hydrolysis and the autocatalytic effects that accelerate degradation is crucial for designing materials with tailored service lives. This review provides a comparative analysis of how different chemical modifications, particularly cross-linking and chain extension, influence the hydrolysis kinetics and degradation pathways of PLA-based materials. The investigation of these mechanisms is set within the broader context of a thesis examining cross-link effects on crystallization and degradation in PLA research, addressing the critical need for predictive models that can guide material selection and development. By synthesizing experimental data from recent studies and presenting clear methodologies, this work serves as a practical resource for scientists seeking to control PLA degradation through strategic molecular design.
Fundamental Mechanisms of PLA Hydrolysis
Ester Bond Cleavage and Polymer Chain Scission
The hydrolytic degradation of PLA initiates with the penetration of water molecules into the polymer matrix, predominantly attacking the amorphous regions due to their lower packing density compared to crystalline domains [16] [17]. This intrusion leads to the cleavage of ester bonds (-CO-O-) along the polymer backbone through a nucleophilic substitution reaction. Water molecules target the carbonyl carbon of the ester group, resulting in chain scission and the generation of shorter polymer fragments with terminal carboxyl (-COOH) and hydroxyl (-OH) end groups [16]. Research indicates this cleavage occurs through two primary mechanisms: random chain scission, where ester bonds are cleaved at any point along the polymer chain, and end-chain scission, where hydrolysis occurs preferentially at terminal ester bonds [16]. The dominance of each mechanism depends on environmental conditions, with end-chain scission reported to be approximately ten times more frequent than random scission in acidic media [16].
The Autocatalytic Effect
A defining characteristic of PLA hydrolysis is its autocatalytic nature. As ester bonds cleave, they generate new carboxyl end groups that increase the local acidity within the polymer matrix [18] [19] [16]. These acidic groups catalyze further ester bond hydrolysis, creating a self-accelerating degradation cycle. This phenomenon is particularly pronounced in larger, bulkier specimens where the acidic oligomers and monomers produced during degradation cannot readily diffuse out of the polymer matrix, leading to accelerated internal degradation known as "bulk erosion" [19]. In contrast, "surface erosion" occurs when the rate of degradation at the surface exceeds the rate of water diffusion into the bulk, which is more common in thinner specimens or under strongly alkaline conditions [19]. The autocatalytic effect creates a heterogeneous degradation profile, with the interior of a specimen often degrading faster than the surface, potentially leading to sudden mechanical failure as the internal structure compromises while the surface remains apparently intact [19].
Table 1: Key Characteristics of PLA Hydrolytic Degradation Mechanisms
| Characteristic | Random Chain Scission | End-Chain Scission | Autocatalytic Bulk Erosion |
|---|---|---|---|
| Site of Attack | Ester bonds at any position along polymer chain | Ester bonds adjacent to chain ends | All accessible ester bonds, preferentially in polymer interior |
| Primary Products | Shorter polymer chains of variable length | Lactic acid monomers | Oligomers, lactic acid, and carboxyl-ended chains |
| Rate Influence | Determines rapid molecular weight decrease | Dominates in acidic environments | Accelerated by accumulation of acidic degradation products |
| Spatial Pattern | Homogeneous throughout water-accessible regions | Concentrated at chain termini | Heterogeneous - faster in specimen interior |
| Molecular Weight Change | Rapid decrease in average molecular weight | Slow decrease in molecular weight with monomer production | Bimodal molecular weight distribution during intermediate stages |
Figure 1: Autocatalytic Hydrolysis Mechanism in PLA. Water penetration initiates ester bond cleavage, generating acidic carboxyl end groups that catalyze further hydrolysis in a self-accelerating cycle.
Experimental Approaches to Studying PLA Hydrolysis
Standard Hydrolysis Protocols and Methodologies
Researchers employ standardized hydrolysis experiments to quantify PLA degradation rates under controlled conditions. A typical protocol involves immersing PLA specimens (films, compressed sheets, or molded items) in aqueous media at precisely controlled temperatures, with periodic sampling to monitor changes in key properties [20] [18] [16]. The selection of hydrolysis medium depends on the intended application—deionized water for fundamental studies, buffer solutions (e.g., phosphate buffer saline, PBS) for biomedical applications, or water-ethanol solutions for food packaging research [20] [19]. Temperature control is critical, with studies often conducted at elevated temperatures (40-95°C) to accelerate degradation for lifetime prediction, while physiological temperature (37°C) is used for biomedical applications [16]. Specimens are typically removed at predetermined intervals, thoroughly dried, and analyzed for molecular weight changes, mass loss, thermal properties, and mechanical performance.
Analytical Techniques for Monitoring Degradation
The progression of hydrolysis is tracked using multiple analytical techniques that provide complementary information. Size Exclusion Chromatography (SEC), also known as Gel Permeation Chromatography (GPC), is the primary method for monitoring changes in molecular weight and molecular weight distribution, offering sensitive detection of chain scission events [18] [19] [16]. Thermal Analysis via Differential Scanning Calorimetry (DSC) tracks changes in glass transition temperature (Tg), melting temperature (Tm), and crystallinity (Xc), which increase during hydrolysis due to enhanced chain mobility and reorganization [16]. Mass Loss measurements quantify the dissolution and release of water-soluble degradation products (monomers and oligomers) from the polymer matrix [18]. Additionally, Nuclear Magnetic Resonance (NMR) spectroscopy, particularly ³¹P-NMR, can elucidate specific degradation mechanisms when specialized additives are involved [19].
Table 2: Core Analytical Methods for Monitoring PLA Hydrolysis
| Analytical Method | Parameters Measured | Information Obtained | Experimental Considerations |
|---|---|---|---|
| Size Exclusion Chromatography (SEC) | Mn, Mw, MWD | Average molecular weights and distribution breadth | Requires appropriate standards; sensitive to sample preparation |
| Differential Scanning Calorimetry (DSC) | Tg, Tm, ΔHm, Xc | Thermal transitions and degree of crystallinity | Heating rate and thermal history significantly affect results |
| Mass Loss Analysis | Residual mass percentage | Release of soluble degradation products | Must carefully dry samples; may plateau before complete degradation |
| Rheological Analysis | Melt viscosity, complex modulus | Changes in processability and mechanical integrity | Measurements sensitive to molecular architecture (branching) |
| NMR Spectroscopy | Chemical structure, end groups | Molecular structure changes, additive degradation | ¹H-NMR for general structure; ³¹P-NMR for phosphite additives |
Figure 2: Experimental Workflow for Studying PLA Hydrolysis. The comprehensive characterization involves multiple analytical techniques after controlled hydrolysis to elucidate degradation mechanisms and kinetics.
Comparative Analysis of Modified PLA Systems
Cross-Linked and Chain-Extended PLA Systems
The incorporation of cross-links or chain extenders significantly alters the hydrolysis behavior of PLA by modifying its molecular architecture. Multifunctional epoxy-based chain extenders, such as Joncryl ADR-4300F, react with carboxyl and hydroxyl end groups of PLA chains, creating branched structures and increasing molecular weight [16]. Studies demonstrate that chain-extended PLA films exhibit significantly slower degradation rates compared to neat PLA across temperatures ranging from 40-95°C [16]. For instance, at 70°C, the time to reach the critical molecular weight for chain entanglement (approximately 8-10 kDa) increased from 10 hours for neat PLA to 25 hours for chain-extended PLA—a 150% improvement in stability [16]. This enhanced resistance to hydrolysis stems from the reduced concentration of accessible carboxyl end groups (which drive autocatalysis) and the increased molecular weight between cross-links, which impedes water penetration and chain mobility.
Elastomer-Modified PLA Blends
Blending PLA with polyolefin elastomers (POEs) represents another modification strategy aimed primarily at improving mechanical properties, but which also influences degradation behavior. Research shows that incorporating POEs (e.g., ethylene-1-octene copolymer) at 15 wt% alongside compatibilizers like POE-g-MA creates a more homogeneous system with improved interfacial compatibility [20]. This morphology reduces the volume and surface area of PLA exposed to aqueous solutions, thereby slowing the hydrolytic degradation rate compared to unmodified PLA [20]. However, the non-biodegradable nature of POEs means that while initial degradation is slower, the resulting composite is no longer fully biodegradable, presenting a trade-off between performance enhancement and environmental impact [20].
Additives for Accelerated Hydrolysis
In contrast to stabilization approaches, some applications benefit from accelerated hydrolysis. Phosphite-based additives, particularly distearyl pentaerythritol diphosphite (Weston 618F), have been shown to significantly enhance hydrolysis rates [19]. Studies demonstrate that PLA compounded with 0.8% phosphite exhibited a 57.7% reduction in molecular weight after 4 days at 58°C, compared to a 31.3% reduction for unmodified PLA [19]. The acceleration mechanism involves the hydrolysis of phosphites to generate acidic compounds (phosphorous acid) that catalyze ester bond cleavage [19]. This approach offers potential for controlling degradation in composting scenarios or for reducing the environmental persistence of PLA litter.
Table 3: Comparative Hydrolysis Performance of Modified PLA Systems
| PLA System | Modification Agent | Key Hydrolysis Findings | Molecular Weight Changes | Activation Energy (Ea) |
|---|---|---|---|---|
| Neat PLA | None | Reference material; rapid autocatalytic degradation | Mn reduced by 31.3% after 4 days at 58°C [19] | 82.7 kJ/mol (40-95°C range) [16] |
| Chain-Extended PLA | Joncryl ADR-4300F (0.5 wt%) | Slower degradation; reduced autocatalysis | Time to reach Mn=10kDa at 70°C: 25h (vs. 10h for neat PLA) [16] | 92.3 kJ/mol (16% increase vs. neat PLA) [16] |
| Elastomer-Modified PLA | POE (15 wt%) + POE-g-MA | Reduced degradation due to lower PLA surface area | Not quantified; degradation rate lower than neat PLA [20] | Not reported |
| Accelerated-Hydrolysis PLA | Distearyl pentaerythritol diphosphite (0.8 wt%) | Significantly accelerated hydrolysis | Mn reduced by 57.7% after 4 days at 58°C [19] | Not reported |
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 4: Key Research Reagents for Studying PLA Hydrolysis
| Reagent/Material | Function in Hydrolysis Research | Specific Examples | Application Notes |
|---|---|---|---|
| PLA Resins | Base material for hydrolysis studies | NatureWorks 2003D (4% D-isomer), 4032D, Luminy L175 (>99% L-isomer) | D-isomer content controls crystallinity; affects degradation rate |
| Chain Extenders | Modify molecular architecture to resist hydrolysis | Joncryl ADR-4300F (epoxy-functional) | Reacts with carboxyl end groups; reduces autocatalytic sites |
| Hydrolysis Accelerators | Enhance degradation rate for controlled lifetimes | Distearyl pentaerythritol diphosphite (Weston 618F) | Hydrolyzes to acidic products that catalyze ester cleavage |
| Compatibilizers | Improve interface in PLA blends | POE-g-MA (polyolefin elastomer grafted with maleic anhydride) | Enhances homogeneity; reduces PLA phase exposure to moisture |
| Hydrolysis Media | Environment for degradation studies | Deionized water, phosphate buffer (PBS), water-ethanol solutions | Choice depends on intended application (medical, packaging) |
| Analytical Standards | Calibration and quantification | Polystyrene standards (SEC), reference materials for DSC | Essential for accurate molecular weight and thermal measurements |
| MT-4 | MT-4, MF:C21H23N5O, MW:361.4 g/mol | Chemical Reagent | Bench Chemicals |
| X77 | X77|Proteinase Inhibitor|For Research Use | X77 is a non-covalent inhibitor for coronavirus main protease (3CLpro) research. This product is for Research Use Only. Not for human use. | Bench Chemicals |
The hydrolytic degradation of PLA, driven by ester bond hydrolysis and autocatalytic effects, can be strategically modulated through various chemical modifications to achieve desired performance profiles. Cross-linked and chain-extended systems demonstrate enhanced resistance to hydrolysis by reducing autocatalytic sites and restricting chain mobility, making them suitable for applications requiring extended functional lifetimes. Conversely, additive-containing systems with controlled catalytic agents enable accelerated degradation, beneficial for reducing environmental persistence. The experimental methodologies and comparative data presented herein provide researchers and drug development professionals with practical tools for designing PLA-based materials with tailored degradation characteristics. As PLA continues to gain prominence across packaging, biomedical, and agricultural sectors, understanding and controlling these fundamental degradation mechanisms remains paramount for optimizing material performance while managing environmental impact.
The manipulation of cross-link density is a fundamental strategy in polymer science for tailoring the properties of materials to meet specific application requirements. Within the context of poly(lactic acid) (PLA) research, understanding the relationship between cross-link density and material performance is crucial for advancing its use in biomedical applications, drug delivery systems, and sustainable materials. This guide provides a comparative analysis of how different cross-linking methods and densities influence the thermal behavior, mechanical performance, and degradation characteristics of PLA-based materials, providing researchers with experimental data and methodologies to inform their material design decisions.
Comparative Analysis of Cross-Linking Methods and Properties
The cross-linking of PLA can be achieved through various methods, each resulting in different network structures and property enhancements. The following sections compare the primary approaches, their resulting material properties, and their implications for practical applications.
Cross-Linking Methodologies and Their Mechanisms
Chemical Cross-Linking involves creating covalent bonds between polymer chains using cross-linking agents or functionalized polymers. One approach utilizes carbodiimide chemistry to couple 4-arm star PLLA prepolymers, creating networks with well-defined molecular weight between crosslinks (Mc) [7]. Another chemical method involves synthesizing crosslinkable poly(lactic acid-co-glycidyl methacrylate) copolymers through ring-opening polymerization, where the incorporated glycidyl methacrylate (GMA) content significantly affects the physical and thermal properties of the resulting copolymers [21].
High-Energy Radiation Cross-Linking employs electron beam or gamma irradiation to generate radicals on polymer chains, which subsequently form cross-links. PLA predominantly undergoes chain scission under ionizing radiation; however, cross-linking can be promoted by adding polyfunctional monomers such as triallyl isocyanurate (TAIC), trimethylolpropane triacrylate (TMPTA), or trimethylolpropane trimethacrylate (TMPTMA) [1]. The cross-linking density can be precisely adjusted by varying the TAIC content and absorbed irradiation dose [22].
Photo-Cross-Linking represents a practical method for curing PLA-based copolymers under mild conditions. The crosslinking of P(LLA-co-GMA) copolymers via this method was shown to be almost complete within 2 minutes, achieving a gel content of 96% [21].
Correlation Between Cross-Link Density and Material Properties
The molecular weight between crosslinks (Mc) is a critical parameter determining the physical properties of cross-linked PLA. Research demonstrates that Mc values significantly influence degradation behavior, with low Mc (1400 g/mol) networks confining degradation to the outer surface, while higher Mc (3500 g/mol) networks exhibit degradation in a 400 μm layer at the surface [7].
Table 1: Comparative Performance of Cross-Linked PLA Systems
| Cross-Linking Method | Cross-Link Agent/Density | Mechanical Performance | Thermal Properties | Degradation Behavior |
|---|---|---|---|---|
| Chemical (Carbodiimide) | Mc = 1400 g/mol | Increased tensile strength vs. linear analogues [7] | Amorphous structure [7] | Surface erosion mechanism [7] |
| Chemical (GMA copolymer) | 19.2 mol% GMA | Compressive stress: 25.5 MPa [21] | Varies from semi-crystalline to amorphous [21] | Thermo-crosslinking at 120°C [21] |
| γ-Ray Irradiation | 10 wt% TAIC, 30 kGy | Shape recovery ratio: 99.5% [22] | Tunable Tg and Tm [22] | Retarded degradation [1] |
| Electron Beam | 3% TAIC, 30-50 kGy | Rubbery and stable above Tm [1] | Improved heat stability [1] | Considerably retarded [1] |
The mechanical properties of cross-linked PLA systems show significant improvements over linear PLA. Chemically cross-linked rigid polyester materials, such as PLLA networks, have been shown to possess comparable or even enhanced tensile strength to their high molecular weight semi-crystalline linear analogues [7]. In radiation cross-linking, the addition of TAIC and appropriate irradiation doses produces materials that become harder and more brittle at low temperatures but remain rubbery, soft, and stable at higher temperatures, even over the melting point (Tm) [1].
The thermal properties and shape memory performance of cross-linked PLA can be precisely tuned through cross-link density control. The incorporation of crosslinking points significantly suppresses cold crystallization and prevents irreversible chain slippage during deformation, resulting in exceptionally high shape recovery ratios (99.5%) and good cycle stability (maintaining 97.9% after three cycles) [22]. These cross-linked systems can be designed as triple-shape memory polymers, utilizing both the glass transition temperature (Tg) and melting temperature (Tm) as switching transitions [22].
The degradation behavior of cross-linked PLA networks differs significantly from linear PLA, transitioning from bulk degradation to a surface erosion mechanism. This shift is particularly evident in networks with low Mc values, where the degradation is confined to the outer surface [7]. The crosslinking process generally leads to a decrease in degradability, which can be advantageous for applications requiring longer implant destruction times or extended drug delivery profiles [1].
Experimental Protocols and Methodologies
Synthesis of Cross-Linked PLA Networks
Chemical Cross-Linking via Carbodiimide Chemistry:
- Synthesize 4-arm hydroxy-terminated star PLLA prepolymers with controlled molecular weights.
- Employ carbodiimide-mediated coupling (using N-(3-dimethylaminopropyl)-N'-ethyl carbodiimide hydrochloride, EDC) to connect the star polymers through succinic anhydride.
- Control the Mc value (1400-3500 g/mol) by adjusting the molecular weight of the prepolymers and the stoichiometry of the reaction.
- Purify the resulting networks to remove any sol fraction and characterize the gel content [7].
Radiation Cross-Linking with TAIC:
- Dry PLA pellets thoroughly in a vacuum oven at 80°C for 12 hours to prevent hydrolysis.
- Premix PLA with triallyl isocyanurate (TAIC) in a Haake mixer at 190°C with a rotation speed of 50 rpm for 10 minutes to form a homogeneous blend.
- Prepare TAIC weight fractions typically ranging from 1% to 10%.
- Hot-press the blends at 190°C under a pressure of 10 MPa to obtain films with uniform thickness (e.g., 300 μm).
- Vacuum seal the specimens and irradiate using a γ-ray source (e.g., 60Co) with doses typically around 30 kGy at room temperature [22].
Characterization Techniques
Gel Content Measurement:
- Weigh the initial dry sample (mâ‚€).
- Immerse the sample in chloroform at room temperature for 48 hours to extract the sol fraction.
- Remove the sample and dry it to constant weight (m₄₈).
- Calculate the gel content using the formula: Gel content = [(m₄₈ - m₀)/m₀] × 100% [22].
Thermal Analysis:
- Perform Differential Scanning Calorimetry (DSC) with a heating rate of 10°C/min from 0°C to 200°C under nitrogen atmosphere.
- Calculate the crystallinity (Xc) using the equation: Xc = (ΔHm - ΔHcc)/ΔHₘⰠ× 100%, where ΔHm is the melting enthalpy, ΔHcc is the cold crystallization enthalpy, and ΔHₘⰠis the standard melting enthalpy for perfectly crystalline PLLA (93 J/g) [22].
- Utilize Dynamic Mechanical Analysis (DMA) to determine the glass transition temperature (Tg) at an oscillation frequency of 5 Hz.
Mechanical Property Assessment:
- For shape memory performance, conduct uniaxial stretching experiments using rectangular specimens.
- Stretch specimens until strain reaches 100% in hot water (80°C), then rapidly cool in cool water (25°C) for 10 seconds to fix the temporary shape.
- Measure shape recovery by reheating the deformed specimens and quantifying the recovery ratio [22].
- Evaluate compressive strength using universal testing machines with constant head speed (e.g., 6 mm/min for hydrogels) [23].
Table 2: Research Reagent Solutions for PLA Cross-Linking
| Reagent | Function | Application Context |
|---|---|---|
| Triallyl Isocyanurate (TAIC) | Polyfunctional cross-linking monomer | Radiation-induced cross-linking [1] [22] |
| Glycidyl Methacrylate (GMA) | Reactive comonomer for copolymer synthesis | Chemical cross-linking via epoxy groups [21] |
| Carbodiimide (EDC) | Coupling agent for carboxyl and hydroxyl groups | Chemical cross-linking of star polymers [7] |
| Trimethylolpropane Triacrylate (TMPTA) | Polyfunctional cross-linking monomer | Electron-beam induced cross-linking [1] |
| Succinic Anhydride | Chain extender/coupling agent | Carbodiimide-mediated network formation [7] |
Cross-Linking Pathways and Property Relationships
The following diagram illustrates the relationship between cross-linking methods, structural parameters, and the resulting material properties in PLA systems:
Cross-Linking Pathways and Property Relationships in PLA: This diagram illustrates how different cross-linking methods influence structural parameters and ultimately determine the final properties of PLA-based materials. The pathways show the progression from method selection to parameter control and resulting performance characteristics.
The systematic correlation between cross-link density and material performance in PLA systems reveals fundamental structure-property relationships that enable precise tuning of thermal, mechanical, and degradation characteristics. Chemical cross-linking methods provide control over network architecture through molecular design, while radiation and photo-cross-linking offer efficient processing routes with spatial and temporal control. The comparative data presented in this guide demonstrates that lower Mc values generally enhance thermal stability, modify degradation from bulk erosion to surface erosion, and improve shape memory performance. These relationships provide researchers with a framework for designing PLA-based materials tailored to specific application requirements in biomedical devices, drug delivery systems, and sustainable materials. Future research directions should focus on developing more precise methods for controlling cross-link density distribution and understanding long-term property evolution during degradation.
Poly(lactic acid) (PLA) has emerged as a leading biodegradable and biobased polymer for applications ranging from biomedical devices to sustainable packaging. However, its widespread adoption is limited by inherent drawbacks, including insufficient heat resistance and poor shape stability at elevated temperatures. Overcoming these limitations is crucial for meeting the performance requirements in demanding fields such as drug delivery and high-temperature packaging. Cross-linking strategies present a powerful approach to enhance PLA's mechanical and thermal properties. Among these, stereocomplexation—the formation of a crystalline structure between poly(L-lactide) (PLLA) and poly(D-lactide) (PDLA)—has garnered significant attention for its ability to substantially improve thermal stability without compromising biocompatibility. This guide provides a comparative analysis of stereocomplex-based cross-linking against other cross-linking methodologies, evaluating their respective impacts on crystallization behavior, heat resistance, and material degradation.
Comparative Analysis of Cross-Linking Strategies for PLA
PLA cross-linking is primarily achieved through three distinct mechanisms: stereocomplexation, chemical cross-linking, and high-energy irradiation. The following sections and comparative table detail the properties and performance of materials produced by each method.
Table 1: Comparison of Cross-Linking Methods for PLA-Based Systems
| Cross-Linking Method | Key Components / Conditions | Melting Temp (Tm) / Heat Resistance | Mechanical Properties | Degradation Resistance | Key Applications |
|---|---|---|---|---|---|
| Stereocomplexation | PLLA + PDLA (Linear or Star-shaped) | ~220-230°C (for Sc crystal) [24]; Vicat Softening Temp: ~64.9°C (at optimal crystallization) [5] | Toughness increased by 40-47x; Elongation at break increased by 35-43x [24] | Strong resistance to hydrolysis [25]; Higher crystallinity decelerates enzymatic degradation [25] | Microwavable packaging [24]; Drug delivery systems [25] |
| Chemical Cross-Linking | EGDMA; PAPI cross-linker [26] [27] | Improved shape stability | Tensile strength increased by 85.5% in BF/SC-PLA composites [27] | N/A | Dual-sensitive hydrogels (temp/pH) [26]; Bamboo fiber composites [27] |
| High-Energy Irradiation | Electron beam/Gamma rays; Cross-linking co-agents (e.g., TAIC) [1] | Rubber-like stability above Tm [1] | Becomes harder and more brittle at low temps [1] | Degradation considerably retarded [1] | Medical implants; Daily-use items [1] |
The data reveals that stereocomplexation uniquely enhances heat resistance by elevating the melting temperature of the crystalline regions. Furthermore, the combination of stereocomplexation with chemical cross-linkers in dual-cross-linked systems leads to synergistic improvements in mechanical strength, as evidenced by the significant increase in tensile strength for bamboo fiber-reinforced composites [27].
Experimental Protocols for Key Methodologies
Forming Stereocomplex Crystals (Sc-PLA)
Objective: To create a stereocomplex PLA structure with enhanced melting temperature and thermal stability.
- Materials: PLLA and PDLA (either linear or star-shaped architectures).
- Procedure:
- Dissolution: Dissolve PLLA and PDLA in a 1:1 mass ratio in a mutual solvent such as tetrahydrofuran (THF) with a typical concentration of 1.0 mg/mL [25].
- Self-Assembly: Allow the solution to stand for a period of 7 days to facilitate the self-assembly and formation of the stereocomplex crystalline structure [25].
- Processing: The solution can be cast into films, or the solid blend can be processed via melt extrusion followed by thermoforming into final products like packaging trays [24].
- Key Characterization Techniques:
- Differential Scanning Calorimetry (DSC): To identify the melting peak of the stereocomplex crystal (~220-230°C), which is distinct from the homopolymer crystal melting peak (~180°C) [24].
- Wide-Angle X-Ray Diffraction (WAXD): To confirm the formation of the stereocomplex crystal structure, which exhibits a characteristic diffraction pattern different from that of homo-crystals [24].
Synthesizing Dual-Cross-Linked Hydrogels
Objective: To prepare a hydrogel with superior mechanical properties and stability by combining physical (stereocomplex) and chemical cross-links.
- Materials: HEMA-terminated PLLA and PDLA macromonomers (HEMA-PLLA/PDLA), temperature-sensitive monomers (MEOâ‚‚MA, OEGMA), pH-sensitive monomer (DEAEMA), chemical cross-linker (ethylene glycol dimethacrylate, EGDMA), and initiator (AIBN) [26].
- Procedure:
- Physical Cross-linking: Dissolve HEMA-PLLA and HEMA-PDLA in THF, subject to low-temperature ultrasonication for 30 minutes to form a stereocomplex physical network, and then evaporate the solvent [26].
- Chemical Cross-linking: Combine the stereocomplexed macromonomers with MEOâ‚‚MA, OEGMA, DEAEMA, EGDMA, and AIBN. Purge the mixture with nitrogen to create an inert atmosphere.
- Polymerization: Seal the reaction vessel and place it in an oil bath at 70°C for 1 hour to initiate free radical polymerization, forming the permanent chemical network [26].
- Key Characterization Techniques:
- Swelling Studies: To evaluate temperature and pH sensitivity by measuring equilibrium swelling ratios in different buffers and temperatures.
- Dynamic Mechanical Analysis (DMA): To assess the mechanical robustness and viscoelastic properties of the dual-cross-linked gel compared to solely physically or chemically cross-linked gels [26].
Cross-Linking via High-Energy Irradiation
Objective: To induce cross-linking in pristine PLA or its blends, improving thermal stability and retarding degradation.
- Materials: PLA polymer and a polyfunctional monomer, such as triallyl isocyanurate (TAIC) or trimethylolpropane trimethacrylate (TMPTMA) [1].
- Procedure:
- Sample Preparation: Mix the PLA with 1-3% of the cross-linking co-agent (e.g., TAIC) to facilitate network formation over chain scission [1].
- Irradiation: Expose the sample to a controlled dose of high-energy radiation, typically an electron beam at doses of 30–50 kGy [1].
- Post-Processing: The cross-linked material can be processed into films or molded parts.
- Key Characterization Techniques:
- Gel Content Analysis: To determine the fraction of insoluble cross-linked material, indicating the efficiency of network formation [1].
- Thermal Analysis (DSC/TGA): To evaluate the enhancement in heat stability and thermal degradation resistance.
Pathway and Workflow Visualization
The following diagram illustrates the strategic decision-making pathway for selecting and implementing a cross-linking method to achieve target material properties, based on the experimental protocols discussed.
Diagram 1: Pathway for Designing Cross-Linked PLA Systems. This workflow guides the selection of primary and complementary cross-linking strategies based on target application requirements.
The experimental workflow for creating a dual-cross-linked system, which yields some of the most synergistic property enhancements, is detailed below.
Diagram 2: Workflow for Dual-Cross-Linked Hydrogel Synthesis. This protocol combines physical stereocomplexation and chemical covalent bonding to create networks with superior properties [26].
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful research and development in cross-linked PLA systems require specific, high-purity reagents and materials. The following table lists key components and their functions.
Table 2: Essential Research Reagents for PLA Cross-Linking Studies
| Reagent/Material | Function/Role | Typical Usage & Notes |
|---|---|---|
| PLLA & PDLA (Linear and Star-shaped) | Enantiomeric polymers that form the stereocomplex crystal backbone. | Use high molecular weight grades for mechanical strength; star-shaped architectures enhance toughness and processability [24]. |
| Triallyl Isocyanurate (TAIC) | Polyfunctional monomer used as a cross-linking co-agent in irradiation. | Applied at 1-3% concentration to promote cross-linking over chain scission during electron beam irradiation [1]. |
| Ethylene Glycol Dimethacrylate (EGDMA) | Chemical cross-linker for creating covalent networks in hydrogels. | Used in free radical polymerization to form permanent, stable chemical bonds within the polymer matrix [26]. |
| Polyaryl Polymethylene Isocyanate (PAPI) | Cross-linker for composites, forming bonds between fillers and polymer matrix. | Improves interfacial adhesion in biomass-filled composites (e.g., bamboo fiber/SC-PLA), significantly boosting tensile strength [27]. |
| Tin(II) Octoate | Catalyst for the ring-opening polymerization of lactide monomers. | Essential for synthesizing PLA macromonomers and star-shaped polymers with controlled architectures [24]. |
| Proteinase K | Enzyme used for in vitro enzymatic degradation studies. | Preferentially hydrolyzes PLLA; used to evaluate and compare degradation rates of different crystalline forms and cross-linked systems [25]. |
| AP39 | AP39|Mitochondria-Targeted H₂S Donor|RUO | |
| ErSO | ErSO|Anticancer Research Compound|RUO | ErSO is a small molecule for research use only (RUO), not for human consumption. It induces tumor regression via a-UPR hyperactivation. Explore its applications. |
This comparison guide demonstrates that stereocomplexation is a uniquely powerful strategy for enhancing the heat resistance and crystalline stability of PLA, primarily through the formation of high-melting-point stereocomplex crystals. When integrated with other methods—such as chemical cross-linking for superior mechanical strength in composites and hydrogels or irradiation for sterilization and stability—researchers can tailor the properties of PLA-based materials to meet specific and demanding application requirements. The choice of method is not mutually exclusive; the most advanced material systems often leverage the synergistic effects of multiple cross-linking mechanisms. The provided experimental protocols and toolkit offer a foundation for researchers in drug development and material science to design and implement these sophisticated cross-linked systems effectively.
Synthesis and Processing: Advanced Methodologies for Cross-Linked PLA in Biomedical and Industrial Applications
Polylactic acid (PLA) is a leading biodegradable and bio-based aliphatic polyester, derived from renewable resources like corn starch, and is recognized as a key material for sustainable plastic solutions. However, its widespread application is hindered by inherent drawbacks, including inadequate impact resistance, brittleness, and poor heat resistance, with a heat resistance temperature typically around 60°C [27]. These limitations restrict its use in demanding applications such as automotive components, durable goods, and thermal insulation. Among various modification strategies, in-situ cross-linking has emerged as a powerful technique to overcome these challenges. This process involves creating a network structure within the polymer blend during processing, significantly enhancing its melt strength, compatibility, and ultimate performance [28] [1].
This guide provides a comparative analysis of prominent in-situ cross-linking techniques for PLA/polymer blends, focusing on their efficacy in improving impact resistance and thermal insulation. We objectively compare the performance of different blend systems and cross-linking strategies, supported by experimental data and detailed methodologies, to serve researchers and scientists in selecting and developing optimal material solutions.
Performance Comparison of Cross-Linked PLA Blends
The following table summarizes key performance metrics for various PLA blends modified with in-situ cross-linking, providing a direct comparison of their effectiveness.
Table 1: Performance Comparison of Cross-Linked PLA Blends
| PLA Blend System & Cross-Linking Strategy | Key Performance Improvements | Impact Strength | Thermal Conductivity (mW/m·K) | Tensile/Flexural Strength | Reference |
|---|---|---|---|---|---|
| PLA/P4HB with In-situ Cross-Linking [28] | Transition from open-cell to closed-cell foam structure; Enhanced heat stability. | Specific impact strength: 26.17 kJ·mâ»Â²/(g·cmâ»Â³) | 31.5 | N/P | [28] |
| PLA/PBAT with Dynamically Cross-Linked ESO (VEC) [29] | Improved compatibility and toughness; Excellent processability. | Significantly improved (specific data in Table 2) | N/P | Maintained or improved | [29] |
| PLA/PBAT with MA Grafting (PBAT-MA) [30] | Enhanced interfacial compatibility and toughness. | 333.9 kJ/m² (+917.3% vs. unmodified) | N/P | Fracture elongation: 358.1% (+450.4%) | [30] |
| Bamboo Fiber/SC-PLA with PAPI Cross-Linking [27] | High heat resistance and improved mechanical properties. | N/P | N/P | Tensile strength: 34.7 MPa (+85.5%) | [27] |
| PLA Cross-Linked via High-Energy Irradiation & TAIC [1] | Improved heat stability and mechanical properties; Retarded degradation. | Becomes harder and more brittle at low temperatures, but soft and stable at higher temperatures. | N/P | Enhanced | [1] |
Abbreviations: N/P - Not Provided in the cited source; P4HB - Poly(4-hydroxybutyrate); PBAT - Poly(butylene adipate-co-terephthalate); ESO - Epoxidized Soybean Oil; VEC - Vulcanized ESO and Citric Acid; MA - Maleic Anhydride; SC-PLA - Stereo-complex PLA; PAPI - Polyaryl Polymethylene Isocyanate; TAIC - Triallyl Isocyanurate.
The data demonstrates that different cross-linking strategies yield distinct performance advantages. The PLA/P4HB foam system shows exceptional promise for thermal insulation applications due to its very low thermal conductivity [28]. Conversely, for impact resistance, the PLA/PBAT-MA system exhibits a dramatic, order-of-magnitude increase in impact strength, making it suitable for high-toughness applications [30].
Detailed Experimental Data and Methodology
Impact Resistance and Toughness
The following table provides quantitative data on the mechanical enhancements achieved through specific cross-linking protocols.
Table 2: Enhancement of Impact Resistance and Toughness
| Blend System | Compatibilizer/ Cross-Linking Agent | Key Processing Parameters | Resulting Mechanical Properties | Reference |
|---|---|---|---|---|
| PLA/PBAT | Epoxidized Soybean Oil (ESO) & Anhydrous Citric Acid (CA) | Dynamic cross-linking; VEC R-value of 0.1 | Significantly improved toughness and impact resistance. | [29] |
| PLA/PBAT | Maleic Anhydride (MA) | Melt-grafting; MA: 2 wt%, BPO initiator: 1 wt%; Twin-screw extruder at 125-135°C | Impact strength: 333.9 kJ/m² (917.3% increase); Fracture elongation: 358.1% (450.4% increase). | [30] |
| Bamboo Fiber/ SC-PLA | Polyaryl Polymethylene Isocyanate (PAPI) | Cross-linking during extrusion (200-220°C) and injection molding (220°C) | Tensile strength: 34.7 MPa (85.5% increase vs. unmodified composite). | [27] |
Thermal Insulation and Stability
In-situ cross-linking significantly influences the thermal properties of PLA blends, particularly by enhancing thermal stability and enabling the formation of insulating foam structures.
Foam Structure and Thermal Insulation: An in-situ cross-linking strategy applied to PLA/P4HB blends drastically improved their foaming behavior. The technique enhanced melt strength and crystallization behavior, causing the foam to transition from an open-cell to a closed-cell structure. This resulted in a volume expansion ratio (VER) of 35.4 and a cell density of 2.0 × 10â· cells/cm³, which are 16.1-fold and 9.1-fold increases, respectively, compared to the unmodified foam. This fine closed-cell structure endowed the foam with excellent thermal insulation properties, achieving a thermal conductivity as low as 31.5 mW/m·K and a thermal diffusivity of 8.1 × 10â»â· m²/s [28].
Heat Resistance: For rigid composites, cross-linking improves heat resistance. Using a cross-linking agent like PAPI in Bamboo Fiber/Stereo-complex PLA (SC-PLA) composites creates a robust network that enhances the material's ability to withstand higher temperatures, which is crucial for applications like heat-resistant food packaging [27]. Furthermore, PLA cross-linked with high-energy irradiation in the presence of co-agents like TAIC exhibits much-improved heat stability, remaining soft and stable at temperatures even above its melting point [1].
Experimental Protocols for Key Techniques
Protocol 1: In-situ Cross-Linking for PLA/P4HB Foams
This protocol is designed to produce closed-cell foams with superior impact resistance and thermal insulation [28].
- Primary Materials: Polylactic acid (PLA), Poly(4-hydroxybutyrate) (P4HB).
- Cross-Linking Strategy: Employ an in-situ cross-linking agent to enhance compatibility between PLA and P4HB.
- Procedure:
- Melt Blending: Blend PLA and P4HB with the cross-linking agent in a melt mixer.
- Supercritical Foaming: Process the modified blend using a supercritical foaming technique.
- Analysis: Characterize the foam morphology (cell density, volume expansion ratio) using scanning electron microscopy (SEM). Measure thermal conductivity using a thermal analyzer and impact strength via impact testing.
Workflow for PLA/P4HB Foam Cross-Linking
Protocol 2: Dynamic Cross-Linking of PLA/PBAT with ESO
This method uses dynamic covalent chemistry to toughen PLA/PBAT blends while maintaining processability [29].
- Primary Materials: PLA, PBAT, Epoxidized Soybean Oil (ESO), Anhydrous Citric Acid (CA), Zinc Acetate (Zn(OAc)â‚‚) catalyst.
- Cross-Linking Strategy: Form a dynamic covalent adaptable network (VEC) between ESO and CA via ring-opening and reversible ester exchange reactions.
- Procedure:
- Mixing: Melt-blend PLA, PBAT, ESO, and CA.
- Dynamic Vulcanization: In-situ formation of the VEC cross-linked network within the blend during processing.
- Compatibilization: The VEC network acts as an intermediate phase to improve PLA/PBAT compatibility.
- Testing: Evaluate mechanical properties (tensile, impact) and thermal stability (DSC, TGA).
Protocol 3: Reactive Melt-Grafting of MA onto PBAT
This protocol focuses on improving interfacial compatibility in PLA/PBAT blends by grafting maleic anhydride onto PBAT [30].
- Primary Materials: PBAT, Maleic Anhydride (MA), Benzoyl Peroxide (BPO) initiator.
- Grafting Strategy: Free radical-induced grafting of MA onto the PBAT chain.
- Procedure:
- Drying: Dry PBAT at 70°C for 12 hours.
- Reactive Extrusion: Use a twin-screw extruder to blend PBAT, MA (1-5 wt%), and BPO (1 wt%). Typical extrusion temperatures are 125-135°C, with a screw speed of 60 rpm.
- Blending with PLA: Melt-blend the resulting PBAT-MA graft copolymer with PLA.
- Analysis: Confirm grafting via FTIR. Test mechanical properties and analyze microstructure morphology (SEM).
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for In-situ Cross-Linking of PLA Blends
| Reagent/Chemical | Function in Cross-Linking Process | Typical Application |
|---|---|---|
| Maleic Anhydride (MA) [30] | Monomer for grafting; enhances interfacial compatibility by reacting with terminal hydroxyl groups of polyesters. | Reactive compatibilizer in PLA/PBAT blends. |
| Benzoyl Peroxide (BPO) [30] | Free radical initiator to kick-start the grafting reaction of MA onto polymer chains. | Initiator for melt-grafting reactions. |
| Epoxidized Soybean Oil (ESO) [29] | Bio-based polyepoxide; undergoes ring-opening with acids to form cross-linked networks. | Dynamic cross-linking agent in PLA/PBAT blends. |
| Anhydrous Citric Acid (CA) [29] | Multi-functional acid; reacts with epoxy groups of ESO and catalyzes dynamic ester exchange reactions. | Co-agent and catalyst for dynamic networks. |
| Triallyl Isocyanurate (TAIC) [1] | Polyfunctional monomer; acts as a cross-linking promoter under high-energy irradiation. | Co-agent for radiation-induced cross-linking. |
| Polyaryl Polymethylene Isocyanate (PAPI) [27] | Multi-functional isocyanate; forms urethane linkages with hydroxyl groups, creating cross-links. | Cross-linker for biomass-filled PLA composites. |
| NH-3 | NH-3, CAS:447415-26-1, MF:C28H27NO6, MW:473.5 g/mol | Chemical Reagent |
| PGPC | PGPC, MF:C29H56NO10P, MW:609.7 g/mol | Chemical Reagent |
Cross-Linking Effects on Crystallization and Degradation
In-situ cross-linking induces profound changes in PLA's crystallization behavior and degradation profile, which are critical within the broader thesis of understanding structure-property relationships.
Effects on Crystallization: Cross-linking can directly influence nucleation and crystal growth. In some systems, the cross-linked network can restrict chain mobility, potentially slowing down the overall crystallization rate [31]. However, specific strategies can counteract this. For instance, the formation of stereocomplex (SC) crystallites in blends of PLLA and PDLA can act as highly effective nucleating agents, accelerating the crystallization of PLLA homo-crystals and raising the melting temperature, thereby enhancing heat resistance [27] [31]. Furthermore, in PLA/P4HB blends, cross-linking was shown to improve crystallization behavior, providing additional nucleation sites for foaming [28].
Effects on Degradation: Cross-linking creates a three-dimensional network that slows down the permeation of water and the progression of hydrolysis through the polymer matrix. Consequently, the degradation rate of cross-linked PLA is considerably retarded compared to its linear counterpart [1]. This is a crucial consideration for both biomedical applications, where longer implantation times may be desired, and for durable goods where extended service life is needed. The degradation profile can be tuned by the cross-linking density, allowing researchers to design materials with tailored lifespans [1].
Cross-Link Effects on PLA Properties
In-situ cross-linking is a versatile and powerful strategy for advancing the performance of PLA blends, directly addressing their key weaknesses in impact resistance and thermal management. The comparative data presented in this guide reveals that the choice of blend partner and cross-linking chemistry dictates the final property profile. PLA/PBAT systems modified with MA or ESO are unparalleled for achieving extreme toughness, making them suitable for applications requiring high impact strength. Conversely, PLA/P4HB blends with in-situ cross-linking are ideal for creating high-performance, biodegradable thermal insulation foams. As research progresses, the refinement of these techniques, particularly using bio-derived reagents and dynamic covalent chemistry, will further expand the applications of sustainable PLA-based materials in demanding technological and industrial fields.
In the field of polymer science, the development of multi-phase systems such as polymer blends and composites is a primary strategy for creating materials with targeted properties. However, most polymer pairs are inherently immiscible, leading to weak interfacial adhesion and poor stress transfer between phases, which ultimately results in unsatisfactory mechanical performance. [32] Reactive processing and compatibilization techniques are engineered to overcome these challenges by creating chemical linkages at the interface, thereby stabilizing the phase morphology and enhancing overall material properties. [32] [33] This is particularly critical for advancing the application of materials like poly(lactic acid) (PLA), a leading bioderived polymer. Within the context of a broader thesis on the comparative study of cross-link effects in PLA, this guide objectively compares how different compatibilization and crosslinking strategies—chemical, electron-beam, and gamma irradiation—impact the material's crystallization behavior and degradation profile, providing researchers with experimental data to inform material selection.
Fundamental Principles of Compatibilization
Compatibilization processes are fundamentally based on the improvement of adhesion between phases, the reduction of interfacial tension, and the stabilization of the phase morphology by inhibiting droplet coalescence during subsequent manufacturing processes. [32] In heterogeneous polymer systems, the overall physical-mechanical behavior critically depends on morphology and interfacial adhesion. The mechanical properties are driven by the interphases' ability to transmit stresses from one phase to the other. [32]
There are two primary compatibilization approaches:
- Reactive Compatibilization: This in-situ method involves generating the compatibilizing agent directly from the homopolymers during processing. A coupling reaction occurs at the interface between functional groups on the different polymer chains, creating a block or graft copolymer that is chemically tethered to both phases. [33] This method is often preferred as it can produce more stable interphase products. [32]
- Additive Compatibilization: This method involves adding a pre-synthesized compatibilizer, such as a block or graft copolymer, to the blend. The compatibilizer locates at the interface and interacts with the phases through chain entanglement and physicochemical affinity. [32]
The kinetics of the interfacial reaction are crucial. The coupling reaction must be fast enough to occur during typical processing times (often less than 5 minutes), and the interface needs to be sufficiently covered with the reactively formed copolymer to effectively compatibilize the blend. [33] Furthermore, flow during melt mixing tremendously accelerates the coupling rate, likely due to convection and the continuous creation of fresh interface. [33]
Comparative Analysis of Crosslinking & Compatibilization Methods for PLA
The following sections and tables provide a detailed comparison of three primary strategies for modifying PLA, summarizing their methodologies, key findings, and influences on crystallization and degradation.
Chemical Crosslinking
Chemical crosslinking typically involves using crosslinking agents or catalysts to form covalent bonds between polymer chains. In the context of compatibilizing blends like polyethylene/polystyrene (PE/PS), the Friedel–Crafts alkylation reaction can be used to graft PE chains onto PS, creating a PE-g-PS copolymer in situ. [32]
Table 1: Summary of Chemical Crosslinking via Reactive Compatibilization
| Aspect | Experimental Findings |
|---|---|
| Methodology | Friedel–Crafts alkylation reaction (F–C) in molten state using anhydrous aluminum chloride (AlCl3) catalyst with 0.3 wt% styrene on PE/PS blends. [32] |
| Copolymer Formation | The amount of copolymer formed increases with catalyst concentration. Shorter polymer chains are predominantly located and react at the interphase. [32] |
| Interfacial Adhesion | The critical micelle concentration (cmc) was reached with a very low catalyst concentration (0.3 wt%). The architecture of the in-situ formed copolymer (graft density, chain length) dictates compatibilization efficiency. [32] |
| Mechanical Performance | Compatibilization leads to a remarkable recovery of tensile properties. For instance, tensile strengths of flame-retardant polypropylene samples reduced by the additive were restored to levels similar to pure PP after compatibilization. [34] |
High-Energy Radiation Crosslinking (E-Beam & Gamma)
Crosslinking via high-energy radiation, such as electron-beam (E-Beam) or gamma rays from a Cobalt-60 (60Co) source, is a versatile method that can be performed on pristine polymer. However, PLA predominantly undergoes chain scission under irradiation, so crosslinking is often promoted using polyfunctional monomers. [1]
Table 2: Summary of High-Energy Radiation Crosslinking
| Aspect | E-Beam Irradiation | Gamma (γ) Irradiation |
|---|---|---|
| Methodology | Irradiation of PLA with an electron beam at specified doses (e.g., 30-50 kGy) and temperatures (e.g., 80 °C, above Tg), often in a nitrogen atmosphere. [1] [35] | Irradiation of PLA/TAIC blends using a 60Co source at room temperature with a typical dose of 30 kGy. [22] |
| Crosslinking Agent | Triallyl isocyanurate (TAIC) or trimethylolpropane triacrylate (TMPTA) at 1-10 wt% is common. [1] [22] | Triallyl isocyanurate (TAIC) is highly effective. [22] |
| Gel Content | Gel content (an indicator of crosslinking density) can be precisely adjusted by TAIC content and irradiation dose. For example, a gel weight of up to 40% was reported with 10 wt% TAIC. [22] | Gel content increases with crosslinking agent content and absorbed dose. [22] |
| Effect on Crystallinity | Crystalline domains negatively affect irradiation-induced crosslinking, which occurs predominantly in amorphous regions. A study showed that a starting crystallinity of 13.3% significantly hindered gel formation compared to amorphous samples. [35] | Crosslinking points can suppress cold crystallization and prevent irreversible chain slippage, enhancing shape memory performance. [22] |
| Effect on Degradation | Crosslinked PLA shows considerably retarded degradation compared to non-crosslinked material. [1] | The crosslinking density controls the degradation rate and mechanism. Networks with low molecular weight between crosslinks (Mc) degrade via surface erosion. [7] |
Comparative Data on Crosslink Density and Degradation
The density of the crosslinked network, often expressed as the molecular weight between crosslinks (Mc), is a critical factor determining the degradation profile of PLA.
Table 3: Effect of Crosslink Density on Hydrolytic Degradation of PLLA Networks
| Molecular Weight Between Crosslinks (Mc) | Crystallinity | Degradation Mechanism | Observations |
|---|---|---|---|
| 1400 g/mol | Amorphous | Surface-erosion | Degradation is confined to the outer surface. [7] |
| 2500 g/mol | Amorphous | Surface-erosion | - |
| 3500 g/mol | ~13% | Surface-erosion | Degradation observed in a 400 μm layer at the surface. [7] |
This data demonstrates that a lower Mc (higher crosslink density) results in a more confined surface degradation, which is a key consideration for designing materials for controlled-release drug delivery or tissue engineering scaffolds where predictable degradation is required.
Experimental Protocols
Protocol: Reactive Compatibilization of Polymer Blends
This protocol is adapted from studies on PE/PS blends. [32]
- Material Preparation: Dry homopolymers (e.g., PE and PS) thoroughly before processing to prevent hydrolysis.
- Melt Blending: Process the polymer blend in an internal mixer (e.g., Haake Polylab) or twin-screw extruder at a temperature above the melting points of all components.
- Catalyst Introduction: Introduce the catalyst (e.g., anhydrous AlCl3 wetted in n-hexane for protection) along with a small amount of co-catalyst (e.g., 0.3 wt% styrene) during the melt blending step.
- Reaction Conditions: Maintain the processing for a defined period (typically < 5 minutes) to allow the in-situ coupling reaction (e.g., Friedel–Crafts alkylation) to occur.
- Analysis: Characterize the resulting blend morphology (SEM), interfacial adhesion (mechanical testing), and graft copolymer formation (sol-gel extraction, FTIR).
Protocol: Gamma-Ray Irradiation Crosslinking of PLA
This protocol is adapted from the fabrication of crosslinked PLLA for shape memory applications. [22]
- Blend Preparation: Dry PLLA pellets at 80 °C in a vacuum oven for 12 hours. Premix PLLA with a crosslinking agent (e.g., Triallyl isocyanurate - TAIC) in a melt mixer at 190 °C for 10 minutes to form a homogeneous blend.
- Film Fabrication: Hot-press the blend into films of desired thickness (e.g., 300 μm) at 190 °C under pressure (e.g., 10 MPa).
- Irradiation: Vacuum-seal the specimens and irradiate using a 60Co gamma-ray source at a predetermined dose (e.g., 30 kGy) at room temperature.
- Post-Irradiation Analysis: Determine the gel content via Soxhlet extraction in a good solvent for PLA (e.g., chloroform) to measure the crosslinked fraction. Analyze thermal and mechanical properties (DSC, DMA, tensile testing).
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 4: Key Reagents for Reactive Processing and Crosslinking of PLA
| Reagent/Material | Function in Research | Example Application |
|---|---|---|
| Triallyl Isocyanurate (TAIC) | A polyfunctional monomer that acts as a crosslinking promoter. Its radicals form covalent connections between polymer chains upon irradiation. [1] [22] | Used in E-Beam and γ-ray crosslinking of PLA to enhance gel content and control crosslink density. [22] |
| Anhydrous Aluminum Chloride (AlCl3) | A catalyst for Friedel–Crafts alkylation reactions, facilitating the grafting of polymer chains. [32] | Used for the reactive compatibilization of PE/PS blends to form PE-g-PS copolymer in situ. [32] |
| Trimethylolpropane Triacrylate (TMPTA) | A crosslinking monomer that enhances the efficiency of radiation-induced crosslinking by providing multiple reactive sites. [1] | Applied as an additive in electron-beam irradiation of PLA to promote network formation. [1] |
| Maleic Anhydride Grafted PP (PP-g-MA) | A compatibilizer that reacts with functional groups (e.g., -OH, -NH2) on other polymers, improving interfacial adhesion in blends. [34] | Used to compatibilize polypropylene with intumescent flame retardants, improving mechanical properties and thermal stability. [34] |
| Edds | EDDS Reagent | EDDS (Ethylenediamine-N,N'-disuccinic acid) is a biodegradable chelating agent for environmental and industrial research. For Research Use Only (RUO). |
| Sams | Sams | High-Purity Research Compound | Supplier | Sams is a high-purity research compound for laboratory use. For Research Use Only. Not for human or veterinary diagnostic or therapeutic use. |
This comparison guide demonstrates that the choice of compatibilization or crosslinking strategy for multi-phase systems like PLA has a profound and direct impact on the material's crystallization behavior and degradation profile. Reactive compatibilization excels at creating tailored interfaces in blends, leading to superior mechanical performance. Meanwhile, radiation crosslinking, particularly when employing promoters like TAIC, offers a solvent-free and efficient route to create networks that enhance thermal stability and provide exceptional shape memory properties. The crosslink density (Mc) is a pivotal design parameter, enabling researchers to tune the degradation mechanism from bulk to surface erosion. For researchers and drug development professionals, this data provides a critical foundation for selecting a processing strategy that aligns with the desired performance and life-cycle of the final product, whether the goal is a durable material for medical devices or a predictably degrading matrix for a drug delivery system.
Additive Manufacturing (AM) of polylactic acid (PLA) has transitioned from a prototyping tool to a method for creating functional, load-bearing components in fields such as biomedical engineering and sustainable manufacturing. A significant limitation of pure PLA is its inherent brittleness and limited thermal stability, which restricts its use in more demanding applications. To overcome these constraints, researchers are exploring material modifications, including chemical cross-linking, and process parameter optimizations, such as the strategic use of infill patterns and densities. Cross-linking, particularly through gamma-ray irradiation, introduces covalent bonds between polymer chains, enhancing shape memory performance, and recovery ratios. Concurrently, infill architecture governs the distribution of material within a printed part, directly influencing its mechanical performance, weight, and material efficiency. This guide objectively compares the performance of cross-linked PLA against other alternatives, focusing on the interplay between material structure and printing parameters, framed within the broader context of controlling crystallization and degradation in PLA.
Comparative Analysis of Material and Infill Strategies
Cross-Linking as a Material Enhancement Strategy
Cross-linking modifies the fundamental polymer architecture of PLA. An innovative approach involves using γ-ray-induced crosslinking in miscible PLLA/triallyl isocyanurate (TAIC) blends. The crosslinking density can be precisely tuned by varying the TAIC content and the absorbed irradiation dose [22].
Key Outcomes of Cross-Linking:
- Enhanced Shape Memory: Cross-linked PLLA demonstrates outstanding shape memory properties, achieving a high shape recovery ratio of 99.5% at 10 wt% TAIC content. This performance is maintained over multiple cycles, with a 97.9% recovery ratio after three cycles [22].
- Stability Under Deformation: The crosslinking points effectively suppress chain slippage during deformation. This allows cross-linked PLLA films to maintain a recovery ratio as high as 99.3% even under an extreme strain of 800%, a condition under which conventional linear PLA would fail [22].
- Triple-Shape Memory: The combination of crosslinking points and PLLA crystals can enable triple-shape memory performance, where both the glass transition temperature (Tg) and melting temperature (Tm) act as switching transitions [22].
The Role of Infill Density and Architecture in Pure PLA
For pure, unmodified PLA, the internal structure created during printing is a critical determinant of mechanical performance. Studies systematically varying infill geometry and density show clear trends in tensile properties.
Tensile Strength and Modulus by Infill Type: The table below summarizes experimental data on the tensile properties of pure PLA specimens fabricated with different infill geometries and densities, tested according to ASTM D638 [36].
Table 1: Tensile properties of pure PLA with different infill parameters [36].
| Infill Geometry | Infill Density | Tensile Strength (MPa) | Young's Modulus (MPa) | Yield Strength (MPa) |
|---|---|---|---|---|
| Cubic | 20% | Data Not Provided | Data Not Provided | Data Not Provided |
| 80% | 21.06 | 1414.19 | 15.59 | |
| Gyroid | 20% | Data Not Provided | Data Not Provided | Data Not Provided |
| 80% | 20.53 | Data Not Provided | 15.52 | |
| Linear (Rectilinear) | 20% | 14.75 | Data Not Provided | Data Not Provided |
| 80% | 20.84 | Data Not Provided | 14.30 |
Key Findings on Infill:
- Higher Density Improves Strength: A strong correlation exists between infill density and tensile strength. For example, linear infill strength increases from 14.75 MPa at 20% density to 20.84 MPa at 80% density [36].
- Geometry Influences Stiffness: At high densities (e.g., 80%), the cubic infill pattern demonstrated the highest Young's Modulus (1414.19 MPa), suggesting it may offer superior stiffness compared to gyroid and linear patterns under these specific conditions [36].
- Optimized Infill for Lightweight Structures: Combining topology optimization with variable infill density can achieve significant weight savings ( 16% to 33% ) while maintaining structural integrity, demonstrating the power of architectural design in material extrusion AM [37].
Comparison with Alternative Material Strategies
Other common strategies to enhance PLA properties include copolymerization and reinforcement with fibers.
- Natural Fiber Reinforcement: Reinforcing PLA with agricultural waste like rice husk and rice straw can improve tensile modulus, flexural strength, and impact resistance. However, a common trade-off is a decline in tensile strength at higher fiber loadings (e.g., 5-20 wt%), which is attributed to void formation and insufficient fiber-matrix adhesion if not properly treated [38].
- Dual-Material Composites: Composites like PLA-TPU fabricated via Fused Filament Fabrication (FFF) can achieve tailored mechanical behavior. For instance, specific dual-material distribution patterns can create composites that outperform their parent materials in flexural modulus and strength [39].
Experimental Protocols for Key Methodologies
Fabrication of Cross-Linked PLLA via γ-Ray Irradiation
Objective: To create PLLA shape memory polymers (SMPs) with precisely controlled cross-linking density and enhanced recovery performance [22].
Materials:
- PLLA (e.g., NatureWorks 3001D)
- Cross-linking agent: Triallyl isocyanurate (TAIC)
- Solvent: Chloroform (for gel content analysis)
Protocol:
- Pre-drying: Dry PLLA pellets in a vacuum oven at 80 °C for 12 hours.
- Melt Blending: Premix PLLA and TAIC (e.g., at 1, 3, 5, and 10 wt%) in a melt mixer (e.g., Haake Polylab) at 190 °C with a rotation speed of 50 rpm for 10 minutes to form a homogeneous blend.
- Hot Pressing: Compress the blend into films (e.g., 300 μm thickness) using a hot press at 190 °C and a pressure of 10 MPa.
- Irradiation: Vacuum-seal the specimens and irradiate using a 60Co γ-ray source at a controlled dose (e.g., 30 kGy) at room temperature.
- Characterization:
- Gel Content: Measure the insoluble gel fraction by soaking weighed samples (m₀) in chloroform for 48 hours, re-weighing after drying (m₄₈), and applying the formula: Gel weight (%) = [(m₄₈ - m₀) / m₀] × 100% [22].
- Thermal Analysis: Use Differential Scanning Calorimetry (DSC) to determine thermal transitions and crystallinity (Xc). Heat specimens from 0°C to 200°C at a rate of 10°C/min. Calculate Xc using the equation: Xc = [(ΔHₘ - ΔH꜀꜀) / ΔHₘâ°] × 100%, where ΔHₘⰠis the standard melting enthalpy for 100% crystalline PLLA (93 J/g) [22].
- Shape Memory Testing: Program specimens by stretching to a set strain (e.g., 100%) in a hot water bath (80 °C), then cooling in cool water (25 °C) to fix the temporary shape. Recovery is triggered by re-immersing in the hot bath, and the shape recovery ratio is calculated [22].
Mechanical Testing of 3D-Printed Infill Structures
Objective: To quantitatively evaluate the effect of infill geometry and density on the tensile properties of 3D-printed PLA structures [36].
Materials:
- PLA filament (diameter 1.75 mm)
- Standard tensile specimen model per ASTM D638 Type I
Protocol:
- Specimen Fabrication: Print tensile specimens using an FFF 3D printer (e.g., Bambu Lab P1S) with controlled parameters:
- Nozzle Diameter: 0.4 mm
- Printing Temperature: 220 °C
- Build Plate Temperature: 55 °C
- Layer Thickness: 0.2 mm
- Experimental Variables:
- Infill Geometries: Cubic, Gyroid, Linear (Rectilinear).
- Infill Densities: 20%, 40%, 60%, 80%.
- Constant: Wall thickness (e.g., 1.2 mm with 3 perimeters).
- Conditioning: Condition printed specimens at room temperature for 8 hours before testing to relieve internal stresses.
- Tensile Testing: Perform tests using a universal testing machine (e.g., Instron 5500R) according to ASTM D638.
- Tensile Speed: 5 mm/min.
- Data Recorded: Force and elongation until fracture.
- Data Analysis: Use associated software (e.g., Bluehill) to calculate tensile strength, Young's modulus, and yield strength from the stress-strain data.
Visualizing Workflows and Relationships
Cross-Linked PLA Fabrication and Shape Memory Workflow
The following diagram illustrates the experimental workflow for creating and evaluating γ-ray cross-linked PLLA, highlighting the causal relationships between each stage and the resulting material properties.
Interplay of Infill and Cross-Linking on PLA Properties
This diagram outlines the logical framework for designing 3D-printed PLA components, showing how cross-linking and infill parameters independently and synergistically influence final part properties.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key materials and reagents for cross-linked PLA and infill studies.
| Item | Function / Relevance | Example from Literature |
|---|---|---|
| PLLA (Poly(L-lactic acid)) | Base polymer for cross-link studies; high purity grades ensure consistent results. | NatureWorks 3001D [22]. |
| TAIC (Triallyl isocyanurate) | Cross-linking agent; forms covalent bonds between PLLA chains under irradiation. | Used at 1-10 wt% in PLLA blends [22]. |
| γ-ray Source (e.g., Cobalt-60) | Provides high-energy radiation to initiate the cross-linking reaction in PLLA/TAIC blends. | 60Co source at 30 kGy dose [22]. |
| PLA 3D Printing Filament | Standard material for infill parameter studies; consistency in diameter is critical. | Bambu Lab 1.75 mm diameter PLA [36]. |
| Chloroform | Solvent for gel content analysis; dissolves uncross-linked PLLA chains. | Used to measure gel fraction of cross-linked networks [22]. |
| Natural Fibers (e.g., Rice Husk/Straw) | Sustainable reinforcement to modify PLA's stiffness and toughness. | 5-20 wt% loading, often with NaOH surface treatment [38]. |
| Boma | Boma | High-Purity Research Compound | RUO | Boma for research applications. High-quality, well-documented compound for scientific investigation. For Research Use Only. Not for human or veterinary use. |
| Talc | Talc | High-Purity Powder for Research Use | High-purity Talc for research applications. For Research Use Only (RUO). Not for human or veterinary diagnostic or therapeutic use. |
Poly(lactic-co-glycolic acid) (PLGA) based thermo-sensitive hydrogels represent a cutting-edge platform in controlled drug delivery, merging the biocompatibility and tunable degradation of PLGA with the spatial and temporal control offered by temperature-responsive materials [40]. These "smart" hydrogels undergo reversible sol-gel transitions in response to temperature changes, enabling minimally invasive implantation through injection followed in situ gelation at body temperature [41]. Within the broader context of poly(lactic acid) research, understanding how cross-linking strategies influence crystallization behavior and degradation kinetics is paramount for designing optimized drug delivery systems [42] [43]. The cross-linking methodology directly governs critical performance parameters including hydrogel mesh size, mechanical integrity, drug release profiles, and degradation rates—all essential considerations for researchers and drug development professionals seeking to overcome limitations of conventional drug formulations [40]. This review systematically compares cross-linking strategies for PLGA-based thermo-sensitive hydrogels, providing experimental data and methodologies to guide material selection for specific therapeutic applications.
Cross-Linking Mechanisms in PLGA Thermo-Sensitive Hydrogels
Fundamental Gelation Mechanisms
Thermo-sensitive hydrogels are classified based on their thermal response behavior, primarily determined by their molecular architecture and polymer-solvent interactions [44]. The gelation process is driven by temperature-induced changes in the hydrophilic-hydrophobic balance within the polymer network [41].
Table 1: Classification of Thermo-sensitive Hydrogel Systems
| System Type | Representative Polymers | Gelation Mechanism | Phase Transition Temperature | Key Characteristics |
|---|---|---|---|---|
| LCST Systems | PNIPAm, PLGA-PEG-PLGA | Gel upon heating above LCST | LCST typically 25-37°C | Entropy-driven; hydrophobic aggregation above LCST |
| UCST Systems | Agarose, certain copolymers | Gel upon cooling below UCST | UCST typically below 37°C | Enthalpy-driven; hydrogen bond formation below UCST |
| Micellar Systems | Pluronic F127, Poloxamers | Micelle formation and packing | CMT and CGT ~20-30°C | Dehydration of PPO blocks leading to micellar organization |
The PLGA-PEG-PLGA triblock copolymer system exemplifies a widely studied thermo-sensitive hydrogel with significant drug delivery applications [44]. Its temperature responsiveness occurs through dehydration of the PEG segment upon heating, which enhances hydrophobicity and promotes micelle aggregation into three-dimensional networks [41]. This behavior makes it particularly suitable for biomedical applications where gelation at body temperature is desired.
Comparative Cross-Linking Strategies
Cross-linking strategies for PLGA-based thermo-sensitive hydrogels are broadly categorized into physical and chemical methods, each offering distinct advantages and limitations for drug delivery applications.
Table 2: Comparison of Cross-Linking Strategies for PLGA Thermo-Sensitive Hydrogels
| Cross-Linking Method | Mechanism | Key Characteristics | Impact on Degradation | Effect on Drug Release |
|---|---|---|---|---|
| Physical Cross-linking | Non-covalent interactions (hydrophobic, hydrogen bonding, chain entanglement) | Reversible, mild gelation conditions, injectable | Faster degradation due to reversible bonds | Often burst release; diffusion-controlled |
| Chemical Cross-linking | Covalent bonding (photo-polymerization, chemical cross-linkers) | Permanent networks, higher mechanical strength | Slower, controlled degradation | Sustained release; degradation-controlled |
| Advanced Structures (e.g., Slide-ring polyrotaxane) | Movable cross-links along polymer chains | Extremely stretchable, tough, shear-thinning | Tunable degradation via pulley effect | Controlled release with mechanical stability |
Physical cross-linking involves the formation of three-dimensional networks through reversible, non-covalent interactions between polymer chains, including hydrophobic associations, hydrogen bonding, and physical chain entanglements during gelation [41]. While this method offers excellent biological safety and avoids potentially toxic cross-linking agents, hydrogels formed solely through physical cross-linking often exhibit limited functional properties, including low mechanical strength, weak adhesion, and burst release of encapsulated substances [41].
Chemical cross-linking creates irreversible covalent bonds within the polymer network, significantly enhancing mechanical stability and providing more predictable drug release profiles [41]. A particularly innovative approach incorporates slide-ring polyrotaxane cross-linkers, where α-cyclodextrin molecules can move along polyethylene glycol chains, creating a "pulley effect" that distributes stress evenly throughout the network [45]. This unique architecture results in remarkably stretchable and tough hydrogels that maintain their structural integrity during deformation, addressing a significant limitation of conventional thermo-sensitive hydrogels for drug delivery applications.
The following diagram illustrates the key cross-linking mechanisms discussed:
Analytical Techniques for Characterizing Cross-Linking Effects
Degradation Assessment Methods
Comprehensive characterization of cross-linking effects requires multiple analytical techniques to evaluate physical, chemical, and mechanical property changes during degradation. The American Society for Testing and Materials (ASTM) provides guidelines for degradation assessment, recommending monitoring of mass loss, molar mass changes, and mechanical properties [46].
Table 3: Analytical Techniques for Assessing Cross-Linking and Degradation
| Assessment Category | Techniques | Parameters Measured | Utility in Cross-Linking Evaluation |
|---|---|---|---|
| Physical Characterization | Gravimetric analysis, SEM, swelling studies | Mass loss, surface morphology, erosion patterns, equilibrium swelling | Infers degradation; assesses network structure and porosity |
| Chemical Characterization | FTIR, NMR, SEC, HPLC, Mass spectrometry | Molecular weight changes, chemical structure, degradation by-products | Confirms degradation mechanism and cross-linking integrity |
| Mechanical Characterization | Rheology, tensile testing, compression testing | Storage/loss moduli, tensile strength, elongation at break | Evaluates cross-linking density and network stability |
| Thermal Analysis | DSC, TGA | Glass transition temperature, crystallinity, thermal stability | Tracks crystallization behavior during degradation |
Scanning Electron Microscopy (SEM) plays a pivotal role in characterizing the morphology of PLGA-based systems. Studies have demonstrated that SEM micrographs reveal significant details about surface texture and porosity of PLGA hydrogels, with higher hydrophilicity correlating with more porous structures that facilitate better cell infiltration and nutrient diffusion [40]. Hybrid PLGA nanoparticle/4-arm-PEG hydrogels display well-defined porous networks essential for controlling drug release rates [40].
Differential Scanning Calorimetry (DSC) provides critical information about the crystallinity changes during PLA degradation. As PLA degrades, the degree of crystallinity typically increases because the amorphous regions degrade faster than crystalline domains [42]. This phenomenon occurs due to the less densely packed polymer chains in amorphous regions, allowing easier penetration of degrading agents, and the larger surface area of amorphous domains resulting in greater exposure to the surrounding environment [42].
Experimental Workflow for Cross-Linking Assessment
The following diagram outlines a comprehensive experimental workflow for evaluating cross-linking effects in PLGA-based thermo-sensitive hydrogels:
Cross-Linking Effects on Crystallization and Degradation
Interplay Between Cross-Linking, Crystallization, and Degradation
The relationship between cross-linking density, crystallization behavior, and degradation kinetics represents a critical consideration in PLA research [42] [43]. Cross-linking restricts polymer chain mobility, which typically reduces crystallinity by inhibiting the molecular reorganization necessary for crystal formation [42]. This reduction in crystallinity significantly influences degradation rates, as amorphous regions degrade faster than crystalline domains due to their less densely packed structure and greater exposure to hydrolytic agents [42].
During degradation, a well-documented phenomenon occurs where the degree of crystallinity increases as degradation proceeds, because the amorphous regions are preferentially eroded, leaving behind a more crystalline material structure [42]. This evolving crystallinity subsequently affects further degradation rates, creating a complex feedback loop between material structure and degradation behavior. The specific cross-linking method directly influences this process—chemical cross-linking creates more stable networks that maintain their structure longer during degradation, while physical cross-linking allows for more dynamic reorganization during the degradation process [41].
Quantitative Data on Cross-Linking Effects
Table 4: Cross-Linking Impact on PLGA Hydrogel Properties and Performance
| Cross-Linking Approach | Gelation Time/Temperature | Mechanical Properties | Degradation Time | Drug Release Profile |
|---|---|---|---|---|
| Physical (PLGA-PEG-PLGA) | Sol-gel transition at ~37°C | Low mechanical strength (storage modulus ~102 Pa) | Days to weeks (depending on MW and LA:GA ratio) | Typically biphasic: initial burst followed by sustained release |
| Chemical (UV cross-linked) | Minutes with photoinitiator | Moderate to high strength (storage modulus ~103-104 Pa) | Weeks to months | More sustained, reduced burst effect |
| Ionic-composite | Variable based on ionic strength | Enhanced toughness through ionic interactions | Tunable via cross-linking density | Controlled, often following near-zero-order kinetics |
| Slide-ring polyrotaxane | Temperature-dependent | Extremely stretchable (up to 900% elongation), high toughness | Extended degradation with maintained integrity | Controlled release even under mechanical stress |
The data demonstrates clear trade-offs between different cross-linking strategies. Physical cross-linking offers rapid temperature-responsive behavior but limited mechanical strength, while chemical cross-linking provides enhanced mechanical properties at the cost of more complex fabrication processes [41]. Advanced systems like slide-ring polyrotaxane cross-linkers achieve remarkable mechanical properties while maintaining functionality, with documented elongations up to 912% compared to just 29% for conventional chemically cross-linked systems at similar cross-linker concentrations [45].
Experimental Protocols for Key Methodologies
Synthesis of PLGA-PEG-PLGA Thermo-sensitive Hydrogels
Materials: PLGA-PEG-PLGA triblock copolymer (various LA:GA ratios), phosphate-buffered saline (PBS, pH 7.4), therapeutic agent for encapsulation [41].
Procedure:
- Dissolve PLGA-PEG-PLGA copolymer in PBS at target concentration (typically 10-30% w/v) by stirring at 4°C for 12 hours until clear solution forms.
- For drug-loaded hydrogels, add therapeutic agent during dissolution process and protect from light if necessary.
- Characterize sol-gel transition using test tube inversion method or rheological analysis with temperature ramp.
- Assess gelation temperature by monitoring viscosity change during temperature increase from 4°C to 50°C at rate of 1°C/min.
- For in vitro release studies, inject hydrogel solution into vials, incubate at 37°C for 10 minutes to form gel, then add release medium [41].
Chemical Cross-Linking via Photopolymerization
Materials: PLGA functionalized with polymerizable groups (e.g., acrylated PLGA), photoinitiator (Irgacure 2959 at 0.1% w/v), UV light source (365 nm, 5-10 mW/cm²) [41].
Procedure:
- Prepare acrylated PLGA by reacting PLGA with acryloyl chloride in dichloromethane in presence of triethylamine.
- Dissolve modified PLGA and photoinitiator in solvent to create 10-20% w/v solution.
- For drug incorporation, add therapeutic agent to polymer solution and mix thoroughly.
- Expose solution to UV light for 1-5 minutes to initiate cross-linking while maintaining sterile conditions.
- Wash cross-linked hydrogel extensively to remove unreacted materials and photoinitiator residues.
- Characterize cross-linking density by swelling studies and mechanical testing [41].
Degradation and Release Kinetics Protocol
Materials: Cross-linked hydrogel samples, phosphate-buffered saline (PBS, pH 7.4), sodium azide (0.02% w/v) as antimicrobial agent, appropriate analytical instruments (HPLC, SEM, rheometer) [46].
Procedure:
- Pre-weigh hydrogel samples (Wâ‚€) and measure initial dimensions.
- Immerse samples in PBS with sodium azide at 37°C under gentle agitation.
- At predetermined time points, remove samples in triplicate and gently blot to remove excess surface water.
- Weigh wet samples (Ww) for swelling calculation, then dry to constant weight (Wd) for mass loss determination.
- Analyze release medium for drug concentration using appropriate analytical method (e.g., HPLC).
- Characterize morphological changes via SEM at different degradation stages.
- Monitor mechanical property changes through periodic rheological measurements.
- Calculate key parameters: Mass loss (%) = [(W₀ - Wd)/W₀] × 100; Swelling ratio = Ww/W_d [46].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 5: Key Research Reagents for PLGA Hydrogel Development
| Category | Specific Materials | Function/Application | Considerations |
|---|---|---|---|
| Polymer Systems | PLGA (various LA:GA ratios), PLGA-PEG-PLGA, PEG-based cross-linkers | Base matrix material providing biodegradability and thermo-sensitivity | LA:GA ratio affects degradation rate; molecular weight influences mechanical properties |
| Cross-Linking Agents | Irgacure 2959, glutaraldehyde, carbodiimides, polyrotaxane derivatives | Create network structure determining mechanical and release properties | Cytotoxicity concerns with some chemical cross-linkers; biocompatibility assessment essential |
| Characterization Reagents | PBS, enzyme solutions (proteases, lipases), solvents for extraction | Simulate physiological conditions and accelerate degradation studies | Enzyme selection depends on degradation mechanism; buffer pH affects hydrolysis rates |
| Therapeutic Carriers | Small molecules (triamcinolone), proteins (bevacizumab), nucleic acids | Model drugs for release studies; actual therapeutic payloads | Drug-polymer interactions affect encapsulation and release; stability during processing |
| Analytical Standards | Lactic acid, glycolic acid, drug reference standards | Quantification of degradation products and drug release | Purity critical for accurate quantification; stability-indicating methods required |
| Cpad | Cpad|Co-Precipitated Amorphous Dispersion | Bench Chemicals | |
| Hpph | HPPH (Photochlor)|Second-Generation Photosensitizer | HPPH is a second-generation photosensitizer for cancer photodynamic therapy (PDT) research. It is For Research Use Only. Not for human use. | Bench Chemicals |
Cross-linking strategies fundamentally govern the performance of PLGA-based thermo-sensitive hydrogels for controlled drug delivery. Physical cross-linking methods offer simplicity and reversibility but typically provide limited control over release kinetics and mechanical strength. Chemical cross-linking creates more robust networks with sustained release profiles but may involve more complex fabrication and potential cytotoxicity concerns. Advanced approaches like slide-ring polyrotaxane systems demonstrate exceptional mechanical properties while maintaining drug delivery functionality. Within the broader context of PLA research, understanding how cross-linking density and methodology influence crystallization behavior and degradation kinetics remains essential for designing optimized drug delivery systems. The selection of appropriate cross-linking strategy must balance multiple factors including mechanical requirements, desired release profile, degradation timeline, and biocompatibility needs for specific therapeutic applications. As characterization techniques continue to advance, particularly in real-time degradation monitoring, researchers will gain enhanced capabilities to precisely tailor PLGA-based thermo-sensitive hydrogels for increasingly sophisticated drug delivery applications.
The escalating global plastic crisis, coupled with mounting pressure to transition toward a circular economy, has intensified the search for sustainable material alternatives. Polylactic acid (PLA), a leading biodegradable and bio-based polymer, has emerged as a frontrunner in this endeavor, finding applications from packaging to biomedical devices [47] [48]. However, its wider adoption is constrained by inherent limitations, including insufficient thermal resistance, inherent brittleness, and a degradation rate that can be either too slow or too fast for specific applications [49] [1]. To overcome these challenges, two key modification strategies have been developed: reinforcement with natural fibers and crosslinking. Natural fiber composites (NFCs), derived from plants like jute, flax, and hemp, offer a renewable, biodegradable, and low-energy manufacturing pathway, potentially reducing carbon emissions by up to 80% compared to conventional materials [50]. Crosslinking, whether by chemical means, high-energy irradiation, or photo-crosslinking, enhances PLA's thermal stability and mechanical properties by creating a three-dimensional network within the polymer structure [1]. This review provides a comparative analysis of cross-linked PLA-natural fiber composites, examining their performance against alternative materials, detailing key experimental methodologies, and evaluating their position within the broader context of sustainable material design.
Performance Comparison of PLA Composites
The performance of PLA composites is highly dependent on both the type of reinforcement and the modification techniques employed. The following tables provide a structured comparison of key properties.
Table 1: Comparative Analysis of PLA Composite Properties and Applications
| Material Type | Key Advantages | Key Limitations | Typical Applications | Degradation Profile |
|---|---|---|---|---|
| Neat PLA | High strength, transparency, biocompatibility [51] [48] | Brittle, low thermal resistance, slow degradation rate [49] [1] | Drug delivery, 3D printing filaments, disposable cutlery [48] | Hydrolytic & enzymatic degradation; rate influenced by temperature, humidity, and catalysts [47] |
| PLA with Synthetic Reinforcements (e.g., MWNT, TiOâ‚‚) | Significantly improved crystallization rate & thermal stability [49] | Higher cost, non-renewable, potential ecotoxicity concerns | Advanced engineering composites | Reinforcing particles can optimize and control the degradation rate [49] |
| PLA with Natural Fibers (e.g., Flax, Jute) | Up to 80% carbon reduction, renewable, biodegradable, competitive performance with treatment [50] | Property variability, moisture sensitivity, lower strength than synthetic composites [50] | Automotive interiors, building panels, consumer products [50] | Generally enhanced biodegradability; susceptible to moisture [50] |
| Cross-linked PLA | Improved heat stability, shape memory, rubbery softness at high temperatures [1] | Increased brittleness at low temps, requires crosslinking agents/equipment, reduced degradability [1] | High-temperature applications, medical implants, durable goods [1] | Degradation considerably retarded due to network formation [1] |
| Cross-linked PLA with Natural Fibers | Balanced mechanical/thermal performance, improved fiber-matrix interface, sustainable profile [50] [1] | Complex manufacturing, potential for property variability, cost | Sustainable automotive parts, biodegradable structural components | Tunable degradation rate based on crosslink density and fiber content |
Table 2: Impact of Reinforcing Particles on PLA Crystallization and Degradation [49]
| Particle Type | Optimum Concentration for Crystallization | Effect on Crystallization Rate | Effect on Hydrolytic Degradation |
|---|---|---|---|
| Multi-Walled Carbon Nanotubes (MWNT) | Yes (specific % not detailed) | Significantly improves | Can be controlled |
| Titanium Dioxide (TiOâ‚‚) | Yes (specific % not detailed) | Significantly improves | Can be controlled |
| Graphene Nanoplatelets (GNP) | Yes (specific % not detailed) | Significantly improves | Can be controlled |
| Natural Fibers (e.g., in blends) | Information Not Specified in Search Results | Information Not Specified in Search Results | Information Not Specified in Search Results |
Experimental Insights and Methodologies
Key Experimental Protocols in PLA Research
To generate the comparative data presented, several standardized experimental protocols are critical.
Protocol 1: Investigating Crystallization Kinetics via Differential Scanning Calorimetry (DSC) DSC is a fundamental tool for understanding how additives and crosslinking influence the thermal behavior and crystallinity of PLA composites, which directly affects mechanical properties and degradation rates [49].
- Sample Preparation: Composite samples are compressed into small, uniform masses (typically 5-10 mg) and sealed in standard DSC pans [49].
- Thermal Cycling: The sample is subjected to a controlled temperature program in an inert atmosphere (e.g., nitrogen). A common cycle involves:
- First Heating: Heating from room temperature to above the melting point (e.g., 190°C) to erase previous thermal history [49].
- Cooling: Cooling to a low temperature (e.g., 25°C) at a controlled rate to study crystallization behavior.
- Second Heating: Re-heating to the melting point to analyze the "history-free" thermal properties, including glass transition (Tð‘”), cold crystallization, and melting temperatures [49].
- Data Analysis: Key parameters like the degree of crystallinity are calculated from the melting enthalpy. The analysis reveals how particles like MWNTs or GNPs act as nucleating agents, significantly increasing the crystallization rate [49].
Protocol 2: Assessing Hydrolytic Degradation Behavior This protocol evaluates the environmental stability and biodegradation potential of PLA composites.
- Sample Immersion: Pre-weighed polymer films or specimens are immersed in a solution that simulates a degradation environment, commonly an alkali solution (e.g., pH 12) maintained at a constant temperature (e.g., 60°C) [49].
- Monitoring: Samples are removed at predetermined time intervals, carefully dried, and re-weighed.
- Property Measurement: The percentage weight loss is calculated. Additional analyses, such as gel permeation chromatography (GPC) to track molecular weight decrease and scanning electron microscopy (SEM) to examine surface morphology changes, are conducted to understand the degradation mechanism [49].
- Conclusion: Studies show that the presence of reinforcing particles and the degree of crystallinity significantly affect the water diffusion pathway and hydrolysis rate, allowing for control over the product's lifespan [49].
Protocol 3: Crosslinking via High-Energy Irradiation Electron beam or gamma irradiation is used to induce crosslinking in PLA, often with the aid of crosslinking co-agents.
- Formulation: PLA is compounded with a polyfunctional monomer, such as Triallyl Isocyanurate (TAIC), at an optimal concentration (e.g., 3% by weight) [1].
- Irradiation: The mixture is exposed to a controlled dose of high-energy radiation (e.g., electron beam at 30–50 kGy). The radiation generates radicals on the polymer chains, which then react with the co-agent to form a crosslinked network [1].
- Post-Irradiation Analysis: The efficiency of crosslinking is measured by the gel content. The crosslinked material is characterized by its improved heat stability, retaining structural integrity even above its melting temperature, and its significantly retarded degradation rate [1].
The Scientist's Toolkit: Key Research Reagents and Materials
Table 3: Essential Materials for Cross-linked PLA-Natural Fiber Composite Research
| Material/Reagent | Function in Research | Specific Examples |
|---|---|---|
| PLA Resin | Base polymer matrix; provides biocompatibility and biodegradability. | NatureWorks PLA 4032D [49] |
| Natural Fibers | Renewable reinforcement; improves specific strength, reduces carbon footprint. | Jute, flax, hemp, silk [50] |
| Crosslinking Co-Agents | Polyfunctional monomers that facilitate network formation during irradiation, minimizing chain scission. | Triallyl Isocyanurate (TAIC), Trimethylolpropane Trimethacrylate (TMPTMA) [1] |
| Compatibilizers | Chemicals that improve adhesion and miscibility between hydrophobic PLA and hydrophilic natural fibers. | Maleic Anhydride, Dicumyl Peroxide, Joncryl [51] |
| Bio-based Fillers | Enhance biodegradability, modify mechanical properties, and reduce cost. | Turmeric, cinnamon, coffee ground powder, rice straw [51] |
| Cps2 | Cps2, MF:C38H42N12O10S2, MW:890.9 g/mol | Chemical Reagent |
| Erinacine A | (+)-Erinacin A|High-Purity Reference Standard | (+)-Erinacin A, a cyathane diterpenoid fromHericium erinaceusmycelium. For neuroprotection and oncology research use only. Not for human consumption. |
Visualizing the Cross-Linking and Degradation Pathway
The following diagram illustrates the core conceptual relationship between cross-linking, crystallization, and the resulting material properties, which forms the thesis of this comparative study.
The strategic combination of cross-linking and natural fiber reinforcement presents a viable pathway to engineer high-performance PLA composites that balance mechanical and thermal properties with environmental sustainability. While cross-linking effectively addresses PLA's shortcomings in heat resistance and allows for degradation rate control, the incorporation of natural fibers mitigates cost, reduces the carbon footprint, and maintains the crucial biodegradable character of the material. The future of this field lies in optimizing the synergy between these approaches—developing more efficient bio-based compatibilizers, refining irradiation protocols for natural fibers, and leveraging digital tools like AI and Digital Twins for predictive lifecycle management [50] [51]. As global regulations and consumer preferences continue to shift toward sustainable materials, cross-linked PLA-natural fiber composites are poised to play an increasingly critical role in a circular bio-economy, finding applications from biodegradable structural components to advanced biomedical implants.
Challenges and Solutions: Optimizing Cross-Linking Parameters for Desired Performance Outcomes
Polylactic acid (PLA) is a leading biodegradable polyester, celebrated for its biocompatibility and production from renewable resources. However, its widespread application in demanding sectors such as biomedical devices, high-performance packaging, and advanced drug delivery systems is critically hindered by inherent brittleness and low toughness [52] [53] [54]. To overcome these limitations, two principal modification strategies have been extensively explored: plasticization and cross-linking. While plasticization aims to increase chain mobility to enhance ductility, cross-linking focuses on creating a network structure to improve strength and dimensional stability [52] [55] [22]. This guide provides a comparative analysis of these strategies, focusing on their distinct effects on the crystallization behavior and degradation profile of PLA, which are crucial parameters for material performance and application lifespan.
Fundamental Mechanisms and Material Design
Plasticization: Enhancing Chain Mobility
Plasticization is a technique to improve the flexibility and ductility of a polymer by incorporating additives that reduce intermolecular forces and increase the free volume between chains.
- Mechanism of Action: Plasticizers work by embedding themselves between polymer chains, effectively shielding the polymer-polymer interactions and increasing segmental mobility. This leads to a lower glass transition temperature (Tg) and increased elongation at break [52] [55].
- Material Design and Reagent Solutions: The choice of plasticizer is critical and can be categorized as follows:
- Low-Molecular-Weight Plasticizers: Citrate esters and fatty acid esters are common. They are efficient but can suffer from leaching over time [55].
- Oligomeric and Polymeric Plasticizers: Poly(ethylene glycol) (PEG) and its derivatives (e.g., acryl-PEG) are widely used. They offer better permanence within the PLA matrix due to their higher molecular weight [52] [55].
- Reactive Plasticizers: Epoxidized soybean oil (ESBO) and acryl-PEG can be grafted onto the PLA backbone via reactive extrusion. This approach, often initiated by peroxides like dicumyl peroxide, minimizes leaching and provides a stable plasticizing effect [55] [56].
Cross-Linking: Creating Network Structures
Cross-linking involves forming chemical bonds between polymer chains, creating a three-dimensional network that restricts large-scale chain motion.
- Mechanism of Action: Cross-links act as permanent physical entanglements. They prevent irreversible chain slippage during deformation, which enhances elastic recovery, toughness, and thermal stability. The cross-linking density directly governs the balance between stiffness and toughness [57] [22].
- Material Design and Reagent Solutions: Several methods are employed to cross-link PLA:
- Irradiation Cross-Linking: Gamma or electron-beam irradiation in the presence of cross-linking agents like triallyl isocyanurate (TAIC). The radiation induces radical formation, leading to covalent bonds between PLA chains and the multifunctional TAIC [22].
- Chemical Cross-Linking: ESBO can act as a compatibilizer and cross-linker in PLA blends. The epoxy rings of ESBO can react with the end groups of PLA or other polymers (e.g., P(3HB-co-4HB)), forming graft copolymers and cross-linked networks that enhance interfacial adhesion and toughness [56].
The following diagram illustrates the fundamental mechanisms and workflows for both plasticization and cross-linking strategies.
Comparative Performance Analysis
The effectiveness of plasticization and cross-linking is quantified through key performance indicators, including mechanical properties, thermal behavior, and crystallization kinetics. The tables below summarize experimental data from key studies.
Table 1: Mechanical and Thermal Properties of Plasticized PLA
| Plasticizer/System | PLA Matrix | Elongation at Break (%) | Tensile Strength (MPa) | Glass Transition (Tg) | Key Findings | Ref. |
|---|---|---|---|---|---|---|
| PEG (Physical Blend) | Not Specified | Significant Increase | Moderate Loss | Decrease | Improved ductility; potential for leaching. | [52] |
| Acryl-PEG (Grafted) | NatureWorks 4042D | 91% (Cycle 1) to 127% (Cycle 5) | Not Specified | Decrease | Reactive extrusion prevented leaching; properties stable upon recycling. | [55] |
| ESBO in PLA/P(3HB-co-4HB) | NatureWorks 4032D | Optimal at 5 wt% ESBO | Maintained | Not Specified | ESBO acted as a compatibilizer and cross-linker, enhancing interfacial adhesion and toughness. | [56] |
| PLA/PCL Copolymer | PLLA | ~800% | No Deterioration | Not Specified | Copolymerization created a flexible, shear-thinning material with high elasticity. | [52] |
Table 2: Mechanical and Thermal Properties of Cross-Linked PLA
| Cross-linking System | PLA Matrix | Key Performance Metrics | Key Findings | Ref. |
|---|---|---|---|---|
| γ-Irradiation with TAIC | NatureWorks 3001D | Shape Recovery Ratio: 99.5% (10 wt% TAIC); Stability: 97.9% after 3 cycles. | Cross-linking points suppressed cold crystallization and prevented irreversible chain slippage, enabling extreme deformations (up to 800% strain). | [22] |
| ESBO in PLA/P(3HB-co-4HB) | NatureWorks 4032D | Significant enhancement in impact toughness and elongation at break. | ESBO's epoxy rings underwent ring-opening reactions, forming cross-linked structures and graft copolymers that improved compatibility. | [56] |
Table 3: Comparative Effects on Crystallization and Degradation
| Property | Plasticization | Cross-Linking |
|---|---|---|
| Crystallization Rate | Increase. Enhanced chain mobility accelerates crystallization kinetics. Grafted acryl-PEG remarkably improved nucleation and crystallization rate under isothermal and non-isothermal conditions [55]. | Variable. Cross-linking points can interrupt chain reorganization, potentially suppressing crystallization (e.g., cold crystallization). However, they can also act as nucleating agents [22]. |
| Overall Crystallinity | Increase. The increased mobility allows chains to arrange into crystalline structures more easily [54] [55]. | Decrease. The covalent network restricts the large-scale motion needed to form well-ordered crystalline lamellae [57] [22]. |
| Degradation Profile | Accelerated. Reduced Tg and increased hydrophilicity (e.g., with PEG) can facilitate water ingress, accelerating hydrolytic degradation [52] [54]. | Slowed & Controlled. The network structure can hinder water penetration and chain disentanglement, leading to a slower and more controlled degradation process [22]. |
Experimental Protocols for Key Studies
Reactive Plasticization Protocol
- Objective: To create a permanently plasticized PLA with enhanced ductility and stable properties upon thermo-mechanical recycling.
- Materials: PLA (NatureWorks 4042D), acryl-PEG (Mn ≈ 480 g/mol), free-radical initiator Luperox 101 (L101).
- Methodology:
- Reactive Extrusion: Dry PLA pellets are mixed with acryl-PEG and L101 in a twin-screw extruder. The process parameters (e.g., temperature profile, screw speed) are set to facilitate the free-radical grafting reaction, where acryl-PEG grafts onto the PLA backbone.
- Injection Molding: The extruded material is pelletized and then injection molded into standard test specimens (e.g., tensile bars).
- Reprocessing Simulation: To simulate recycling, the specimens are subjected to multiple extrusion and injection molding cycles (e.g., up to 5 cycles).
- Characterization: After each cycle, specimens are analyzed for molecular weight (size exclusion chromatography), thermal properties (Differential Scanning Calorimetry), tensile behavior, and morphology (Atomic Force Microscopy) [55].
Irradiation Cross-Linking Protocol
- Objective: To fabricate cross-linked PLA with superior shape memory properties and high recovery under large deformations.
- Materials: PLLA (NatureWorks 3001D), triallyl isocyanurate (TAIC).
- Methodology:
- Melt Blending: Pre-dried PLLA is melt-blended with TAIC (1-10 wt%) in an internal mixer at 190°C to create a homogeneous blend.
- Hot Pressing: The blend is hot-pressed at 190°C under pressure to form films of a specific thickness (e.g., 300 μm).
- Irradiation: The sealed films are irradiated using a ^60^Co γ-ray source at a controlled dose (e.g., 30 kGy) at room temperature. This induces cross-linking via radical mechanisms.
- Characterization: The cross-linked films are evaluated for gel content (to measure cross-linking density), thermal transitions (DSC, DMA), and shape memory performance through cyclic thermo-mechanical tests [22].
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Reagents for PLA Toughening Studies
| Reagent | Function in Research | Key Characteristic |
|---|---|---|
| Poly(ethylene glycol) (PEG) & Derivatives | Acts as a polymeric plasticizer to increase chain mobility and reduce Tg. | Biodegradable, non-toxic, and available in a range of molecular weights. Acryl-PEG allows for reactive grafting [52] [55]. |
| Epoxidized Soybean Oil (ESBO) | Serves as a bio-based plasticizer and compatibilizer; epoxy groups can react to form cross-links. | Multi-functional; enhances toughness in PLA blends and improves interfacial adhesion [56]. |
| Triallyl Isocyanurate (TAIC) | Used as a cross-linking agent (coagent) in irradiation-induced cross-linking. | Polyfunctional; enhances the efficiency of cross-link formation upon radiation, allowing for precise control of network density [22]. |
| Dicumyl Peroxide (DCP) & Luperox 101 (L101) | Free-radical initiators used in reactive extrusion processes. | Generates radicals at processing temperatures to initiate grafting (plasticization) or cross-linking reactions [55] [56]. |
| Poly(ε-caprolactone) (PCL) | Used as a flexible polymer for blending or creating block copolymers with PLA. | Biodegradable polyester that imparts flexibility and improves impact strength [52]. |
| RW | RW, MF:C17H24N6O3, MW:360.4 g/mol | Chemical Reagent |
The choice between plasticization and cross-linking for mitigating PLA's brittleness is application-dependent, governed by a clear trade-off between ductility and structural integrity. Plasticization is the preferred strategy when the primary goal is to maximize elongation and flexibility, often at the expense of some tensile strength and with a potential acceleration of the degradation rate. Cross-linking is the method of choice for applications requiring enhanced toughness, superior shape memory, controlled degradation, and high dimensional stability, particularly under load. The advent of multi-functional additives like ESBO, which can bridge the gap between these strategies by offering both plasticizing and cross-linking effects, points toward a future of hybrid approaches. Researchers can leverage these insights to tailor PLA properties precisely, enabling its successful deployment in advanced biomedical and engineering applications.
The controlled degradation of poly(lactic acid) (PLA) and similar polymers is a critical focus in materials science, particularly for applications in drug delivery and tissue engineering. A significant challenge in this field is managing the interplay between polymer crystallization, degradation kinetics, and drug release profiles. The inherent acidic microenvironment that develops during polymer hydrolysis can lead to undesirable burst release of encapsulated agents and autocatalytic degradation, ultimately compromising device performance and longevity. This comparative guide examines how crosslinking strategies fundamentally alter the crystallization behavior and degradation kinetics of PLA-based systems. By synthesizing current research data, we provide a structured analysis of methodologies to overcome these persistent challenges, enabling researchers to select appropriate strategies for specific application requirements.
Fundamental Mechanisms and Challenges
The Acidic Microenvironment and Burst Release Phenomenon
Poly(lactic acid) degrades through hydrolysis of its ester bonds, generating lactic acid and other acidic byproducts. In bulk materials, these acidic compounds become trapped within the polymer matrix, creating a localized acidic microenvironment. This phenomenon is particularly pronounced in larger devices with low surface-to-volume ratios. The acidic conditions can accelerate the degradation process in a self-propagating "autocatalytic" effect, leading to premature structural failure and rapid, uncontrolled release of encapsulated drugs known as "burst release" [58].
The crystallinity of PLA plays a contradictory role in this process. While crystalline regions generally provide structural integrity and slow degradation, the relationship is not straightforward. Research shows that initially amorphous PLA samples degrade faster than pre-crystallized samples even when they achieve similar crystallinity during the degradation process, suggesting that initial chain organization and the concentration of catalytic chain ends in amorphous regions significantly influence degradation kinetics [58].
Crosslinking as a Regulatory Strategy
Crosslinking introduces covalent bonds between polymer chains, creating a three-dimensional network that fundamentally alters material properties. In PLA, crosslinking affects both the crystallization behavior and degradation profile by restricting chain mobility, influencing water permeability, and altering the distribution of acidic degradation products. These networks can suppress strain-induced crystallization during deformation, which is crucial for maintaining predictable degradation rates in applications requiring mechanical stress [22]. The crosslinking density serves as a critical parameter that can be precisely tuned to achieve desired release profiles and degradation timelines.
Comparative Analysis of Crosslinking Strategies
Crosslinking Methodologies and Their Impact
Table 1: Comparison of PLA Crosslinking Strategies and Their Effects on Material Properties
| Crosslinking Strategy | Mechanism | Effect on Crystallinity | Degradation Rate Control | Burst Release Mitigation |
|---|---|---|---|---|
| γ-Irradiation with TAIC | Free radical formation and covalent bond creation between chains | Suppresses cold crystallization; stabilizes crystalline regions as physical crosslinks | Significantly extends degradation time; prevents rapid chain slippage | Excellent: Maintains 97.9% shape recovery after 3 cycles even at 800% strain [22] |
| Chemical Crosslinking | Multifunctional agents creating covalent networks between polymer chains | Can restrict crystal growth; depends on crosslink density | Moderate to high control; depends on crosslink density | Good: Prevents sudden structural collapse but may not eliminate initial burst |
| Stereocomplex Formation | Co-crystallization of PLLA and PDLA creating dense physical networks | Increases crystallinity and melting temperature | Slows degradation due to reduced water permeability | Good: Creates more diffusion barriers but limited by blending homogeneity |
| Reactive Extrusion | Grafting of plasticizers or agents onto PLA backbone during processing | Can enhance nucleation and crystallization rate while maintaining stability | Can accelerate initial degradation if chain scission occurs | Moderate: Improved stability over conventional blending but dependent on agent |
Quantitative Comparison of Degradation Behavior
Table 2: Experimental Data on Crosslinked PLA Performance Under Accelerated Degradation
| Material Formulation | Initial Crystallinity (%) | Molecular Weight Retention After 120h (%) | Shape Recovery Ratio (%) | Release Profile Modulation |
|---|---|---|---|---|
| Linear PLLA | ~1 (amorphous) | <50% | <70% (at 100% strain) | High burst release (>60% in 24h) |
| PLLA/1% TAIC Crosslinked | ~25 | ~75 | >95% | Moderate burst (~40% in 24h) |
| PLLA/5% TAIC Crosslinked | ~30 | ~85 | >98% | Low burst (~20% in 24h) |
| PLLA/10% TAIC Crosslinked | ~35 | ~90 | 99.5% | Minimal burst (<10% in 24h) [22] |
| Annealed PLLA (A100) | ~32 | ~70 | N/A | Variable depending on crystal morphology |
The data reveal that γ-irradiation crosslinking with TAIC demonstrates superior performance in controlling both degradation and release profiles. Systems with higher TAIC content (5-10%) and corresponding crosslinking density maintain significantly higher molecular weight retention during accelerated degradation testing, indicating robust resistance to hydrolytic chain scission. This structural preservation directly correlates with improved control over release profiles, minimizing the initial burst effect that plagues many conventional PLA formulations [22].
Experimental Protocols for Crosslinking and Characterization
γ-Ray Crosslinking with TAIC
Materials Preparation:
- PLLA resin (e.g., NatureWorks 3001D) should be dried in a vacuum oven at 80°C for 12 hours to remove moisture
- Triallyl isocyanurate (TAIC) as crosslinking agent
- Prepare PLLA/TAIC blends with varying TAIC concentrations (1, 3, 5, and 10 wt%) using a Haake mixer at 190°C with a rotation speed of 50 rpm for 10 minutes
- Hot-press blends at 190°C under 10 MPa pressure to form films of 300 μm thickness
- Vacuum-seal specimens and irradiate using a 60Co γ-ray source with a dose of 30 kGy at room temperature [22]
Characterization Methods:
- Gel Content Measurement: Determine crosslinking efficiency by measuring insoluble fraction after 48 hours of chloroform immersion using the formula:
Gel weight (%) = (m₄₈ - m₀)/m₀ × 100%
where m₀ is initial dry weight and m₄₈ is weight after swelling [22]
- Thermal Analysis: Use Differential Scanning Calorimetry (DSC) with heating/cooling rate of 10°C/min under nitrogen flow to determine crystallinity:
Xc = (ΔHₘ - ΔH꜀꜀)/ΔHₘⰠ× 100%
where ΔHₘ is melting enthalpy, ΔH꜀꜀ is cold crystallization enthalpy, and ΔHₘⰠis standard melting enthalpy (93 J/g for PLLA) [22]
- Shape Memory Testing: Conduct uniaxial stretching experiments at 80°C (above Tg) with 100% strain, followed by cooling to 25°C to fix temporary shape, then reheating to measure recovery ratio [22]
Accelerated Degradation Testing
Protocol:
- Prepare specimens of standardized dimensions (e.g., 15 mm × 3 mm × 0.3 mm)
- Expose to accelerated degradation conditions (70°C, 95% relative humidity) for predetermined intervals
- At each time point, remove specimens for:
- Molecular weight analysis via Gel Permeation Chromatography (GPC)
- Tensile testing to monitor mechanical property changes
- Mass loss measurements
- pH monitoring of surrounding medium [58]
Data Interpretation:
- Monitor changes in molecular weight distribution to identify random chain scission versus selective degradation
- Correlate crystallinity changes with degradation rates using DSC and WAXD
- Compare degradation profiles of initially amorphous versus crystalline samples to understand the role of initial morphology [58]
Visualization of Mechanisms and Workflows
Crosslinking Impact on Degradation Pathways
(Caption: Comparative degradation pathways of linear versus crosslinked PLA systems. Crosslinking creates a protective network that mitigates the autocatalytic effect and prevents burst release.)
Experimental Workflow for Crosslinking Evaluation
(Caption: Comprehensive experimental workflow for developing and characterizing crosslinked PLA systems, from material preparation through degradation analysis.)
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagents and Materials for PLA Crosslinking Studies
| Reagent/Material | Function/Application | Key Characteristics | Representative Examples |
|---|---|---|---|
| PLLA Resin | Primary polymer matrix | High molecular weight (Mw ~150,000-200,000 g/mol), controlled D-isomer content | NatureWorks 4042D, 3001D [22] |
| Triallyl Isocyanurate (TAIC) | Crosslinking agent for irradiation | Trifunctional allyl compound, forms radicals under γ-irradiation | Sigma-Aldrich, Sinopharm Chemical Reagent [22] |
| γ-Ray Source | Crosslinking initiation | 60Co source, precise dose control (typically 30 kGy) | Industrial irradiation facilities [22] |
| Poly(ethylene glycol) methyl ether acrylate (acryl-PEG) | Reactive plasticizer | Enhances chain mobility, grafts onto PLA backbone | Sigma-Aldrich (Mn ≈ 480 g molâ»Â¹) [55] |
| Free-radical Initiator | Initiates grafting reactions | Peroxide-based (e.g., 2,5-dimethyl-2,5-di(tert-butylperoxy)hexane) | Luperox 101 (L101) [55] |
| Chloroform | Solvent for gel content measurement | High purity HPLC grade for accurate swelling measurements | Various suppliers [22] |
| Polyvinyl Alcohol (PVA) | Stabilizer for nanoparticle formation | Prevents aggregation during nanoprecipitation | MW: 30,000–70,000 [59] |
Crosslinking strategies represent a powerful approach for controlling PLA degradation rates and overcoming challenges associated with acidic microenvironments and burst release. The comparative data presented in this guide demonstrates that γ-irradiation crosslinking with TAIC provides exceptional control over degradation kinetics while maintaining mechanical integrity under demanding conditions. These strategies enable researchers to precisely tune material performance for specific applications from long-term implants to controlled drug delivery systems.
Future research directions should focus on optimizing crosslinking density gradients within devices, developing more precise spatial control of crosslinking patterns, and creating multi-stimuli responsive systems that adapt to biological environments. Additionally, further investigation is needed to understand the long-term biological interactions of crosslinked PLA degradation products and their clearance pathways. As analytical techniques continue to advance, particularly in real-time monitoring of degradation phenomena, researchers will gain deeper insights into the fundamental mechanisms governing PLA performance, enabling the rational design of next-generation biodegradable devices with precisely controlled lifespans and release profiles.
The fabrication of cross-linked poly(lactic acid) (PLA) presents a complex paradox: the very networks that enhance thermal and mechanical properties often adversely processability, particularly by increasing melt viscosity and challenging melt strength control. This guide objectively compares the performance of cross-linked PLA against other alternatives, framing the analysis within a broader thesis on the comparative effects of cross-linking on crystallization and degradation. Cross-linking, while improving heat stability and mechanical strength, fundamentally alters the viscoelastic behavior of PLA melts, leading to distinct processing challenges not encountered with its linear or blended counterparts [1] [60]. The following sections provide a comparative analysis supported by experimental data, detailed methodologies, and key reagents to aid researchers in navigating these fabrication hurdles.
Comparative Performance of PLA Materials
The following tables summarize the key properties and processing challenges of cross-linked PLA compared to other common modification strategies.
Table 1: Comparison of Key Properties and Processing Parameters
| Material Type | Typical Tensile Strength (MPa) | Typical HDT (°C) | Melt Strength | Melt Viscosity | Key Processing Challenge |
|---|---|---|---|---|---|
| Cross-linked PLA | 47 - 60 [7] [60] | > 60 [1] | High [61] | High / Complex [1] | High zero-shear viscosity; potential for degradation during cross-linking [61] [1] |
| Linear PLA | 50 - 60 [60] | ~60 [60] | Low [61] | Low | Low melt strength and elasticity; limits film blowing and foaming [61] |
| PLA Blends (e.g., with PBAT, PP) | Variable | Variable | Moderate | Moderate | Managing immiscibility; requires compatibilizers [51] [62] [60] |
| Chain-Extended PLA | High [61] | Improved | High [61] | High | Similar to cross-linking; structure difficult to control [61] |
Table 2: Impact of Cross-Linking on Material Behavior
| Property | Impact of Cross-Linking | Experimental Evidence |
|---|---|---|
| Zero Shear Viscosity | Strong increase [61] | Co-polymerization with 0.4 mol% diethylglycolide increased zero-shear viscosity significantly [61]. |
| Melt Elasticity | Strong increase [61] | Evidenced by enhanced melt strength and elongational viscosity [61]. |
| Crystallization | Can be promoted by low/moderate crosslinking [63] | Onset crystallization temperature increased; however, perfection of crystal lamellas became inferior with high crosslink density [63]. |
| Hydrolytic Degradation | Rate is reduced and mechanism shifts towards surface erosion [7] | Network with Mc of 1400 g/mol showed surface degradation, while linear PLA degraded via bulk erosion [7]. |
| Thermal Stability | Significantly improved [1] | Cross-linked samples remain stable at temperatures over Tm, exhibiting rubbery properties [1]. |
Experimental Protocols for Cross-Linked PLA Characterization
Rheological Analysis
Objective: To quantify the melt strength and viscoelastic properties of cross-linked PLA. Methodology:
- Small Amplitude Oscillatory Shear (SAOS): Measurements are performed using a parallel-plate rheometer. After compression molding samples into discs (e.g., 8mm diameter, 1mm thick) and drying, tests are conducted under nitrogen atmosphere. A strain sweep (e.g., 0.1-10%) determines the linear viscoelastic region. Frequency sweeps (e.g., 0.1-100 rad/s) are then performed at the processing temperature (e.g., 185°C) to obtain storage (G') and loss (G'') moduli [61].
- Extensional Viscosity: An Extensional Viscosity Fixture (EVF) is used for strain-controlled stretch experiments. Compression-molded rectangular samples are pre-stretched to compensate for thermal expansion, relaxed to remove residual stress, and then stretched at constant Hencky strain rates (e.g., 0.1 to 3.5 sâ»Â¹) to measure elongational viscosity [61].
Assessment of Cross-Link Density
Objective: To determine the degree of cross-linking, a critical parameter influencing melt behavior. Methodology:
- Gel Fraction Measurement: A known weight (Wâ‚€) of the cross-linked sample is immersed in a good solvent for uncross-linked PLA (e.g., chloroform) for 24 hours. The insoluble gel fraction is then dried to a constant weight (Wâ‚). The gel content is calculated as (Wâ‚ / Wâ‚€) × 100% [63] [1].
- Swelling Test: The same dried gel is weighed to obtain its swollen mass (W_s) after solvent immersion. The degree of swelling is calculated, with higher cross-link densities resulting in lower swelling ability [63].
Monitoring Hydrolytic Degradation
Objective: To evaluate how cross-linking alters the degradation profile of PLA. Methodology: Samples are immersed in a degradation medium such as phosphate-buffered saline (PBS) at 37°C or 0.1M NaOH for accelerated testing. The samples are removed at set intervals, rinsed, dried, and analyzed for mass loss, changes in thermal properties (via DSC), and mechanical property retention [7].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for Cross-Linked PLA Fabrication and Analysis
| Reagent | Function | Key Characteristic / Consideration |
|---|---|---|
| Triallyl Isocyanurate (TAIC) | Crosslinking Co-agent | Polyfunctional monomer; used in irradiation and peroxide-induced crosslinking to enhance efficiency and gel content [63] [1]. |
| Dicumyl Peroxide (DCP) | Radical Initiator | Used in chemical crosslinking to initiate radical formation on polymer chains [63]. |
| Stannous Octoate | Polymerization Catalyst | Catalyzes ring-opening polymerization of lactide; typical monomer:catalyst ratio of 2500:1 [61]. |
| Hexamethylene Diisocyanate (HMDI) | Crosslinker / Compatibilizer | Used to compatibilize interfaces (e.g., between PLA and cellulose nanofiber); forms carbamate linkages [64]. |
| Diethylglycolide (EG) | Co-monomer | Incorporated via ROP to enhance melt strength by expanding polymer coils and increasing entanglements [61]. |
| Epoxidized Soybean Oil (ESO) | Bio-based Plasticizer/Crosslinker | Can react with PLA's hydroxyl/carboxyl groups, improving toughness and flexibility [62]. |
| Chloroform | Solvent | Used for dissolving PLA, gel fraction tests, and precipitation of polymers [61] [7]. |
Workflow and Relationships in Cross-Linked PLA Fabrication
The following diagram illustrates the core decision-making workflow and logical relationships involved in selecting and fabricating a cross-linked PLA system to manage melt strength and viscosity.
Diagram 1: Cross-linked PLA Fabrication Workflow
Effectively managing melt strength and viscosity during the fabrication of cross-linked PLA requires a nuanced understanding of the trade-offs between final material performance and processability. Cross-linked PLA exhibits superior thermal stability, mechanical strength, and a shift towards a more controllable surface erosion degradation mechanism compared to linear PLA and simple blends. However, these advantages come at the cost of significantly increased melt viscosity and more complex processing behavior. The choice of cross-linking method—be it chemical, radiative, or co-polymerization—directly influences the network structure and, consequently, the melt properties. Successful fabrication therefore hinges on the precise optimization of cross-link density and the application of appropriate rheological characterization techniques to guide processing parameters, enabling researchers to harness the full potential of cross-linked PLA for advanced applications.
Enhancing Cell Compatibility and Reducing Immunogenicity in Cross-Linked PLGA Systems
Poly(lactic-co-glycolic acid) (PLGA) is a cornerstone biodegradable polymer in biomedical engineering, valued for its biocompatibility and tunable degradation properties [65]. However, upon implantation, its interaction with the biological environment can trigger adverse immune responses and compromise cell compatibility, potentially leading to inflammation, fibrotic encapsulation, and failure of the medical device or drug delivery system [66]. Cross-linking is a powerful strategy to modulate the physical and chemical properties of PLGA, directly influencing its crystallization behavior, degradation kinetics, and subsequent biological performance [56]. Within the context of a broader thesis on comparative study of cross-link effects in poly(lactic acid) research, this guide objectively compares the performance of various cross-linked PLGA systems against alternative modification strategies. It provides a structured overview of the key experimental data and methodologies used to evaluate their success in enhancing cell compatibility and reducing immunogenicity for researchers and drug development professionals.
Comparative Analysis of PLGA Modification Strategies
The following table summarizes the core objectives, common methodologies, and key findings related to enhancing PLGA for biomedical applications.
Table 1: Comparison of PLGA Modification Strategies for Improved Biocompatibility
| Strategy | Primary Objective | Key Methodologies | Impact on Cell Compatibility & Immunogenicity |
|---|---|---|---|
| Cross-linking with ESBO [56] | Enhance mechanical toughness and interfacial compatibility in polymer blends. | Blending PLA/P(3HB-co-4HB) with Epoxidized Soybean Oil (ESBO); FT-IR, DSC, SEM analysis. | Improved compatibility reduces phase separation, potentially leading to a more consistent degradation profile and reduced inflammatory stimulus. |
| End-Group Functionalization [67] | Modulate biomolecule-polymer interactions and degradation kinetics. | Synthesis of PLGA with -OH, -NH2, or -COOH terminal groups; in vitro release studies and macrophage uptake assays. | PLGA-NH2 showed reduced hydrophobicity, faster degradation, and significantly reduced phagocytic clearance by macrophages. |
| Surface PEGylation [68] | Reduce opsonization, extend systemic circulation time, and improve stability. | Conjugating Poly(Ethylene Glycol) (PEG) to PLGA nanoparticle surface; ligand anchoring for active targeting. | PEG corona minimizes protein adsorption and immune recognition, decreasing immunogenicity and promoting "stealth" properties. |
| Composite Fabrication (ZIF-8) [69] | Impart multifunctional properties (e.g., osteogenesis, antibacterial). | Incorporating Zeolitic Imidazolate Framework-8 (ZIF-8) into PLGA/Collagen nanofibers via electrospinning. | Sustained release of Zn2+ provides antimicrobial activity, preventing infection-related inflammation and promoting osteogenic differentiation. |
Experimental Protocols for Key Methodologies
Polymer Synthesis and Formulation
1. End-Group Functionalization of PLGA Polymers [67]
- Materials: PLGA polymers (50:50 lactide:glycolide, ~5 kDa) with native carboxyl (-COOH) end groups are used as a starting point. Functionalized polymers with amine (-NH2) or hydroxyl (-OH) end groups are commercially sourced or synthesized via controlled ring-opening polymerization.
- Microparticle (MP) Formulation: A double emulsion-solvent evaporation technique (w/o/w) is employed.
- First Emulsion: 200 µL of an aqueous solution containing the therapeutic agent (e.g., peptide or protein) is added to 200 mg of PLGA dissolved in 4 mL of dichloromethane (DCM). The mixture is sonicated at 55% amplitude for 10 seconds to form a water-in-oil (w/o) emulsion.
- Second Emulsion: The primary emulsion is transferred to 60 mL of a 2% polyvinyl alcohol (PVA) aqueous solution and homogenized at 3000 rpm for 1 minute to form a double (w/o/w) emulsion.
- Solvent Evaporation & Harvesting: The resulting emulsion is stirred in a 1% PVA solution (80 mL) at 600 rpm for 3 hours on ice to allow DCM evaporation. MPs are collected by centrifugation, washed, and lyophilized.
2. Fabrication of PLGA/Col/ZIF-8 Composite Nanofibrous Membranes [69]
- Electrospinning Solution Preparation: PLGA is dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) at 20% (w/v). Type I collagen (Col) is added at an optimized weight ratio of 3:100 (Col:PLGA). ZIF-8 nanoparticles (1 wt%) are dispersed in HFIP via sonication, followed by the addition of PLGA and Col.
- Electrospinning Process: The solution is loaded into a syringe with a 23 G flat-tipped needle. Electrospinning is performed at an applied voltage of 10–15 kV, a solution flow rate of 0.5 mL/h, and a needle-to-collector distance of 15 cm. The resulting nanofibrous membranes are collected and dried.
Characterization and In Vitro Evaluation
1. Assessing Physical Properties and Degradation [67] [46] [69]
- Surface Wettability: Water contact angle (WCA) is measured using an optical tensiometer. A lower WCA indicates higher hydrophilicity.
- Thermal Properties: The glass transition temperature (Tg) is determined using Differential Scanning Calorimetry (DSC) by heating polymer samples from -20°C to 150°C at a rate of 10°C min-1.
- In Vitro Degradation: MPs or films are incubated in phosphate-buffered saline (PBS, pH 7.4) at 37°C. Samples are periodically removed, washed, and dried.
- Mass Loss: Determined gravimetrically.
- Molecular Weight Change: Analyzed using Size Exclusion Chromatography (SEC) or by monitoring intrinsic viscosity.
- Morphological Analysis: Surface and bulk morphology are examined using Scanning Electron Microscopy (SEM).
2. Evaluating Cell Compatibility and Immunogenicity [66] [67] [69]
- Cell Viability and Proliferation: Assessed using assays like Live/Dead staining and Cell Counting Kit-8 (CCK-8) on relevant cell lines (e.g., fibroblasts, osteoblasts).
- Macrophage Uptake: Bone marrow-derived macrophages are incubated with PLGA MPs. Phagocytic clearance is quantified via flow cytometry or fluorescence microscopy.
- Immune Cell Activation: The interaction of PLGA nanoparticles with antigen-presenting cells (e.g., dendritic cells) and subsequent T-cell activation are monitored using flow cytometry and cytokine ELISA kits.
- Osteogenic Differentiation: For bone-relevant applications, Alkaline Phosphatase (ALP) activity and Alizarin Red S (ARS) staining are used to quantify osteogenic differentiation.
Data Visualization and Workflows
PLGA Modification and Immune Evasion Pathways
The following diagram illustrates the logical workflow for developing and evaluating enhanced PLGA systems.
PLGA Nanoparticle Immune Interaction Mechanism
This diagram outlines the immunological mechanisms of PLGA nanoparticles and how modifications alter their fate in vivo.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for PLGA Compatibility and Immunogenicity Research
| Reagent / Material | Function / Role | Application Example |
|---|---|---|
| PLGA (varied LA:GA ratios) [65] [70] | Base biodegradable polymer; properties tuned by copolymer ratio. | 50:50 PLGA for faster degradation/drug release; 75:25 for more sustained release. |
| Epoxidized Soybean Oil (ESBO) [56] | Bio-based cross-linker and compatibilizer. | Enhances interfacial adhesion and mechanical toughness in PLA/PLGA blends. |
| Functionalized PLGA (e.g., PLGA-NHâ‚‚) [67] | Modulates surface charge, degradation, and biomolecule interactions. | Reduces hydrophobic interactions with proteins and minimizes macrophage uptake. |
| Poly(Ethylene Glycol) (PEG) [68] | "Stealth" polymer; reduces opsonization and improves nanoparticle circulation time. | Conjugated to PLGA surface (PEGylation) to minimize immune recognition. |
| Zeolitic Imidazolate Framework-8 (ZIF-8) [69] | Functional nanofiller; provides antimicrobial and osteoinductive properties. | Incorporated into PLGA fibers for controlled Zn²⺠release in guided bone regeneration. |
| Type I Collagen [69] | Natural biopolymer; improves hydrophilicity and cell adhesion. | Blended with PLGA to create composite electrospun membranes that mimic the extracellular matrix. |
| Tin(II) 2-ethylhexanoate (Sn(Oct)â‚‚) [71] | Common catalyst for ring-opening polymerization of lactide/glycolide. | Synthesizing PLGA copolymers with controlled molecular weights and architectures. |
| Polyvinyl Alcohol (PVA) [67] [68] | Surfactant and stabilizer in emulsion-based particle formation. | Used as a continuous phase in double emulsion-solvent evaporation methods for microparticle fabrication. |
Optimizing Cross-Linker Concentration and Distribution for Homogeneous Network Formation
In poly(lactic acid) (PLA) research, achieving a homogeneous polymer network is paramount for tailoring material properties to specific applications, from biomedical devices to sustainable packaging. The formation of this ideal network is critically dependent on two factors: the precise concentration of cross-linking agents and their uniform distribution throughout the polymer matrix. An optimal concentration ensures the formation of sufficient cross-links to enhance properties like heat resistance and mechanical strength without causing excessive brittleness or hindering processability. Simultaneously, a uniform distribution prevents the formation of weak spots and localized stress concentrators, ensuring consistent performance throughout the material. This guide provides a comparative analysis of mainstream cross-linking strategies for PLA, evaluating their effectiveness in achieving this delicate balance and its subsequent impact on crystallization behavior and material degradation.
Comparative Analysis of PLA Cross-Linking Strategies
The following section objectively compares the performance of three primary cross-linking methods for PLA, focusing on their respective capabilities and limitations in achieving a homogeneous network.
Table 1: Performance Comparison of PLA Cross-Linking Strategies
| Cross-Linking Method | Typical Cross-Linker & Concentration | Key Performance Advantages | Limitations & Network Heterogeneity Risks | Best-Suited Applications |
|---|---|---|---|---|
| High-Energy Irradiation (e.g., E-beam, γ-ray) | Triallyl isocyanurate (TAIC): 1-3 wt% [1] [22] | Effective heat resistance improvement; Enhanced shape memory properties (up to 99.5% shape recovery) [22] | High risk of chain scission competing with cross-linking; Can require precise control of radiation dose [1] | Medical devices, heat-resistant components, shape memory polymers [1] [22] |
| Reactive Extrusion (Chemical) | Peroxides (e.g., DCP) with co-agents (e.g., TAIC) | Industrially scalable process; Potential for significant increase in melt strength [72] | Complex competition between scission and cross-linking; Mixing efficiency critical for uniform distribution [72] | Film blowing, foaming, packaging where high melt strength is required [72] |
| Synergistic Nucleation (Physical) | Co-nucleating agents (e.g., EBH & TMC-306): ~1.5 wt% total [73] | Excellent film-forming properties & thickness uniformity; Forms beneficial nanocrystal networks [73] | Does not create covalent cross-links; Property enhancement is thermally limited | High-clarity packaging films, applications requiring superior optical properties and uniformity [73] |
Key Insights from Comparative Data
The data reveals a clear trade-off between the strength of covalent cross-links and the ease of achieving homogeneity. While high-energy irradiation and reactive extrusion can profoundly improve thermal and mechanical properties, they involve complex chemical reactions that are inherently difficult to control. The competition between chain scission and cross-linking during these processes is a significant source of network heterogeneity, potentially leading to inconsistent material performance [1] [72]. In contrast, synergistic nucleation employs a physical strategy to create a network of nanocrystals that act as physical cross-links. This method demonstrates superior performance in achieving film uniformity, as the small-molecule nucleating agents can disperse more readily to form a homogeneous network without the risk of chain degradation [73].
Experimental Protocols for Cross-Linker Evaluation
To reliably compare the effects of different cross-linking strategies and optimize their concentration, researchers employ a set of standardized experimental protocols. The workflow for a comprehensive study typically involves sample preparation, cross-linking, and a multi-faceted characterization of the resulting polymer network.
Diagram 1: Experimental workflow for evaluating cross-linker concentration and distribution in PLA, from sample preparation to multi-faceted network characterization.
Detailed Experimental Methodologies
Sample Preparation and Cross-Linking
- Melt Blending: Pre-dry PLA pellets in a vacuum oven at ~80 °C for 12 hours to prevent hydrolysis. Melt-blend PLA with the cross-linker (e.g., TAIC, EBH/TMC-306) in an internal mixer (e.g., Haake Polylab) at 190 °C with a rotation speed of 50 rpm for 10 minutes to ensure homogenization [73] [22].
- Hot Pressing: Compress the blended material into films of uniform thickness (e.g., 300 μm) using a hot press at 190 °C under a pressure of 10 MPa [22].
- Cross-Linking via Irradiation: Seal the samples in vacuum bags and irradiate using a
γ-raysource (e.g.,60Co) at a specified dose (e.g., 30 kGy) at room temperature [22]. For reactive extrusion, the process involves melting PLA and injecting peroxides and cross-linking co-agents directly into the extruder barrel [72].
Quantitative Analysis of Cross-Linking
- Gel Content Measurement: This is a direct method to quantify the formation of an insoluble network. Weigh a dried sample (m₀), submerge it in a suitable solvent (e.g., chloroform) for 48 hours, then extract and dry the insoluble portion to obtain its weight (m₄₈). Calculate the gel content as:
Gel Content (%) = (m₄₈ / m₀) × 100%[22]. - Differential Scanning Calorimetry (DSC): Analyze crystallization behavior by subjecting samples to a heat-cool-heat cycle (e.g., 0 °C to 200 °C at 10 °C/min). Determine the degree of crystallinity (Xc) using the formula:
X_c (%) = [(ΔH_m - ΔH_cc) / (ΔH_m₀ × w)] × 100%, where ΔHm is melting enthalpy, ΔHcc is cold crystallization enthalpy, ΔHm₀ is the theoretical melting enthalpy of 100% crystalline PLA (93 J/g), and w is the weight fraction of PLA in the composite [22].
The Scientist's Toolkit: Key Research Reagents and Materials
Table 2: Essential Reagents for PLA Cross-Linking Research
| Reagent/Material | Function in Research | Key Considerations |
|---|---|---|
| Triallyl Isocyanurate (TAIC) | Multi-functional cross-linking co-agent for irradiation and peroxide-induced systems [1] [22]. | Miscibility with PLA is excellent, which promotes homogeneous distribution [22]. Optimal concentration is often 1-3 wt% [22]. |
| Hydrazide Nucleators (TMC-306) | Organic nucleating agent that self-assembles to form crystalline nuclei, creating a physical network [73]. | Can be used in synergy with other agents (e.g., EBH) to form dense nanocrystal networks for improved properties [73]. |
| Amide Nucleators (EBH) | Self-assembles into nano-fibrous networks via hydrogen bonding, providing numerous nucleation sites [73]. | Has a lower melting point (~150 °C); the temperature window for inducing crystallization during cooling can be narrow [73]. |
| Dicumyl Peroxide (DCP) | Common peroxide used in reactive extrusion to generate primary radicals for initiating cross-linking [72]. | Its decomposition rate and concentration must be carefully controlled to favor cross-linking over the competing chain scission reaction [72]. |
| Chloroform | Solvent for gel content extraction experiments to quantify the insoluble, cross-linked network fraction [22]. | High purity is required to avoid interference with measurements. Proper fume handling and disposal are necessary. |
Mechanistic Insights: Scission vs. Cross-Linking
A fundamental challenge in chemically cross-linking PLA is the competition between the desired cross-linking reaction and the detrimental chain scission. Understanding this mechanism is key to optimizing conditions for a homogeneous network.
Diagram 2: The competitive reaction pathways during PLA cross-linking, showing the desired cross-linking and the competing chain scission that leads to degradation.
The reaction begins with the generation of primary radicals, either from decomposed peroxides or high-energy irradiation. These radicals abstract hydrogen atoms from the PLA backbone, creating unstable mid-chain radicals (MCRs). The fate of these MCRs determines the outcome:
- Path A (Chain Scission): The MCR undergoes β-scission, breaking the polymer chain. This reduces the molecular weight and compromises mechanical properties [72].
- Path B (Natural Cross-linking): Two MCRs can recombine, forming a covalent bond between polymer chains. However, this reaction is often inefficient.
- Path C (Co-agent Cross-linking): The MCR reacts with a multi-functional co-agent like TAIC, which has multiple reactive sites. This efficiently bridges adjacent PLA chains, creating a homogeneous 3D network. The use of co-agents is crucial to promote Path C over Path A [1] [72].
Optimizing cross-linker concentration and distribution is a central challenge in advancing PLA material science. The choice between chemical cross-linking (irradiation, reactive extrusion) and physical networking (synergistic nucleation) dictates the property profile and ultimate application of the material. Chemical methods, while powerful, require meticulous control to mitigate chain scission and achieve homogeneity. Physical methods offer a more straightforward path to uniformity for specific applications like packaging films. Future research will continue to refine these protocols, with a growing emphasis on model-based tools that can predict the complex scission-crosslinking competition during processes like reactive extrusion, thereby accelerating the development of high-performance, reliably homogeneous PLA materials [72].
Performance Benchmarking: Comparative Analysis of Cross-Linked PLA Systems Across Applications
Understanding and predicting the degradation kinetics of poly(lactic acid) (PLA) is fundamental to advancing its application in biomedical devices and controlled drug delivery systems. Degradation kinetics modeling provides a critical framework for predicting mass loss and drug release profiles, enabling researchers to design optimized materials without solely relying on extensive and time-consuming experimental trials. For semi-crystalline polymers like PLA, the interplay between hydrolysis-induced chain scission, crystallization, and oligomer diffusion creates a complex degradation pattern that challenges simple predictive models. The presence of cross-links further influences this dynamic by restricting polymer chain mobility, thereby affecting both crystallization behavior and degradation pathways. This review systematically compares the predominant mathematical frameworks used to model PLA degradation, evaluates their capabilities in capturing crystallization-degradation interactions, and summarizes the experimental data validating their predictions. By synthesizing insights from recent research, this guide aims to equip scientists with the knowledge to select appropriate modeling strategies for specific application contexts, ultimately accelerating the development of advanced PLA-based technologies.
Comparative Analysis of Mathematical Frameworks for Degradation Kinetics
Table 1: Comparison of Primary Mathematical Frameworks for PLA Degradation Kinetics
| Model Type | Core Mathematical Formulation | Key Input Parameters | Predictive Outputs | Applicability to Semi-Crystalline PLA & Cross-linking Effects |
|---|---|---|---|---|
| Bulk Erosion (First-Order Kinetics) | ∂Ce/∂t = -kâ‚Ce [74] |
Initial molecular weight, degradation rate constant (kâ‚) | Molecular weight loss over time, mass loss | Limited; does not explicitly account for crystallinity or cross-linking. Best for amorphous polymers. |
| Autocatalytic Model | ∂Rs/∂t = kâ‚Ce + kâ‚‚Ce (Col/(1-Xc))^0.5 [75] [74] |
Crystallinity (Xc), oligomer concentration (Col), rate constants (kâ‚, kâ‚‚) | Heterogeneous degradation, internal oligomer buildup, molecular weight distribution | High; explicitly incorporates crystallinity and autocatalytic effects. Can be adapted for cross-link density. |
| Multi-Scale Models (e.g., MS-CMCA) | Combines Monte Carlo for chain scission, Fick's law for diffusion, and cellular automata for cavity formation [75] | Initial molecular weight distribution, crystallinity, copolymer ratio, device geometry | Molecular weight change, crystallinity evolution, weight loss, inner pore structure | Very High; captures stochastic chain scission and its coupling to crystallization. Ideal for complex device geometries. |
| Reaction-Diffusion Finite Element Analysis (FEA) | ∂Col/∂t = ∂Rol/∂t + ∇·(D∇Col) coupled with mechanical properties [76] [74] |
Diffusion coefficient (D), mechanical stress (σ), crystallinity | Local degradation profiles, stent diameter change, mechanical integrity over time | Very High; directly incorporates stress and crystallinity. Suitable for evaluating cross-linking impact on performance. |
The autocatalytic model is particularly effective for semi-crystalline PLA as it quantifies how the degree of crystallinity (Xc) slows the diffusion of oligomers, trapping them inside the polymer matrix and accelerating internal degradation [74]. Multi-scale models and reaction-diffusion FEA provide the most comprehensive tools for studying cross-link effects, as they can simulate how cross-links impede chain mobility, thereby influencing both the rate of crystallization and the diffusion of water and oligomers [75] [76].
Experimental Protocols for Model Validation
Validating mathematical models requires robust experimental data quantifying degradation against time. The following protocols are foundational for generating such data.
Protocol for In Vitro Hydrolytic Degradation
This standard protocol monitors mass loss and molecular weight change under simulated physiological conditions.
- Materials Preparation: Prepare PLA films or devices with specified geometries (e.g., 2 mm thick plates). Key material parameters include the initial number-average molecular weight (
Mn₀), theD-lactic acid content(to control initial crystallinity), and cross-link density [75] [5]. - Incubation: Immerse samples in a phosphate-buffered solution (PBS) at a constant physiological temperature (typically 37 °C). The pH is often maintained at 7.4, though it can be left un-buffered to study autocatalytic effects [75].
- Sampling and Analysis: At predetermined time intervals, remove samples (n=3-5) from the incubation medium and analyze them.
- Molecular Weight: Use Gel Permeation Chromatography (GPC) to determine the instantaneous number-average molecular weight (
Mn(t)) and its distribution over time [75] [43]. - Mass Loss: Carefully rinse, dry, and weigh the samples to track residual mass. The
normalized massis calculated asm(t)/mâ‚€[75]. - Crystallinity: Employ Differential Scanning Calorimetry (DSC) to measure the evolving
degree of crystallinity (Xc(t))during degradation [5] [74]. - Oligomer Release & pH: The pH of the aging medium can be monitored, and the eluted oligomers can be quantified using techniques like high-performance liquid chromatography (HPLC) [75].
- Molecular Weight: Use Gel Permeation Chromatography (GPC) to determine the instantaneous number-average molecular weight (
Protocol for Monitoring Drug Release Kinetics
This protocol quantifies drug release profiles from PLA-based carriers, which serve as critical validation for coupled degradation-release models.
- Experimental Setup: Use a USP apparatus (e.g., paddle method) containing a release medium (e.g., PBS at 37°C) under sink conditions [77] [78].
- Drug Loading: Incorporate a model drug (e.g., Theophylline or a fluorescent marker) into the PLA matrix during fabrication [79].
- Sampling and Quantification: At regular intervals, withdraw aliquots from the release medium and analyze them using UV-Vis spectroscopy or HPLC to determine the
cumulative drug release[79] [78]. The receptor medium must be replenished to maintain a constant volume.
The data generated from these protocols—Mn(t), Xc(t), mass loss, and drug release profile—are directly used to fit and validate the parameters of the mathematical models described in Table 1 [75] [74].
Visualization of Model Structures and Workflows
The following diagram illustrates the logical structure and interconnections of a comprehensive multi-scale degradation model, integrating the key concepts from the discussed frameworks.
The workflow demonstrates how modern frameworks like the Multi-Scale Cellular Monte Carlo Automata (MS-CMCA) integrate processes across different scales [75]. The microscopic scale stochastic chain scission events directly influence the mesoscopic scale, where chain mobility leads to crystallization and cavity formation. These morphological changes, in turn, alter the macroscopic scale diffusion coefficients, creating a dynamic feedback loop that governs overall mass loss and drug release [75] [74].
The Scientist's Toolkit: Essential Reagents and Materials
Table 2: Key Research Reagent Solutions for PLA Degradation and Release Studies
| Reagent/Material | Function in Experimental Research | Relevance to Modeling |
|---|---|---|
| Poly(L-lactide) (PLLA) Resins | The primary biodegradable polymer substrate, available in varying molecular weights and D-isomer content to control initial crystallinity [5] [43]. | Defines initial parameters in models: Mnâ‚€, Ceâ‚€, and Xcâ‚€. |
| Phosphate Buffered Saline (PBS) | A standard incubation medium for in vitro degradation studies, simulating physiological pH and ionic strength [75]. | Provides the aqueous environment for hydrolysis in the model. |
| Model Drugs (e.g., Theophylline) | Small molecule active pharmaceutical ingredients used to track release kinetics from PLA matrices [79] [78]. | Provides experimental drug release profile data for model validation. |
| Enzymes (e.g., Proteinase K) | Used to study enzymatic degradation, which can surface-erode PLA, a different mechanism from bulk hydrolysis [77]. | Represents an alternative degradation pathway not covered by standard hydrolysis models. |
| Cross-linking Agents (e.g., Peroxides, Triallyl Isocyanurate) | Used to introduce cross-links into the PLA matrix, restricting chain mobility and altering crystallization and degradation behavior [5]. | The key variable for studying its "cross-link effects on crystallization and degradation," a core thesis context. |
The selection of an appropriate degradation kinetics model for PLA is not a one-size-fits-all endeavor but is dictated by the specific research focus and material complexity. For preliminary studies on amorphous PLA, simple first-order kinetics may suffice. However, for advanced applications like bioresorbable stents or controlled drug release systems where semi-crystalline structure, autocatalysis, and mechanical integrity are critical, more sophisticated frameworks are indispensable. Autocatalytic and reaction-diffusion models provide robust tools for incorporating the profound effects of crystallinity, while multi-scale and FEA approaches offer unparalleled insights into the complex, interdependent processes governing mass loss and drug release. As research into cross-linked PLA progresses, these advanced models will be pivotal in elucidating the structure-function relationships that guide the design of next-generation biodegradable polymers.
Poly(lactic acid) (PLA) is a prominent biodegradable polymer with significant potential in biomedical and structural applications. However, its inherent brittleness and limited heat resistance restrict its utility in load-bearing contexts. This guide provides a comparative analysis of two primary strategies to overcome these limitations: the incorporation of fiber reinforcements and the application of cross-linking techniques. Cross-linking modifies the polymer's network structure, enhancing thermal and mechanical properties, while fiber reinforcement directly improves strength and stiffness. Drawing on current research, we objectively compare the performance of neat PLA against its cross-linked and fiber-reinforced counterparts, presenting quantitative data on mechanical properties, thermal stability, and degradation behavior to inform material selection for advanced applications.
The performance of Poly(lactic acid) (PLA) in load-bearing applications is critically dependent on its crystallinity and degradation profile, which are in turn profoundly influenced by its structural modifications. As a cornerstone of a broader thesis on cross-link effects on crystallization and degradation in PLA research, this guide examines how cross-linking, as a specific material strategy, compares to both neat PLA and fiber-reinforced composites. Neat PLA, while biocompatible and biodegradable, exhibits a tensile strength of 21-60 MPa and a tensile modulus of 0.35-3.5 GPa, but suffers from poor toughness, typically showing less than 10% elongation at break [80]. Its heat deflection temperature is relatively low (53-56°C), leading to potential deformation under elevated temperatures [81]. Cross-linking introduces covalent bonds between polymer chains, creating a network structure that can enhance heat resistance, improve shape stability, and delay hydrolytic degradation [1]. This review synthesizes experimental data to compare these material systems, providing researchers and scientists with a foundational understanding of their relative performance for applications ranging from orthopedic implants to engineering components.
Comparative Mechanical and Thermal Performance
The mechanical suitability of a material for load-bearing applications is determined by its strength, stiffness, and ability to withstand operational temperatures. The following tables summarize key properties of neat, cross-linked, and fiber-reinforced PLA, providing a direct comparison.
Table 1: Tensile and Flexural Properties of PLA Formulations
| Material Formulation | Tensile Strength (MPa) | Tensile Modulus (GPa) | Flexural Strength (MPa) | Flexural Modulus (GPa) | Reference |
|---|---|---|---|---|---|
| Neat PLA | 21.0 - 60.0 | 0.35 - 3.50 | ~85.2 (from manufacturer data) | ~2.38 (from manufacturer data) | [80] [82] |
| PLA with 15% Bamboo Fiber & Cross-linker | 34.7 | Not Specified | Not Specified | Not Specified | [27] |
| Short Carbon Fibre Reinforced PLA (Flat) | 47.9 (from manufacturer); 47.1% increase over neat PLA in study | 4.79 (from manufacturer); 179.9% increase over neat PLA in study | 114 (from manufacturer); 89.75% increase over neat PLA in study | 6.32 (from manufacturer); 230.95% increase over neat PLA in study | [82] |
Table 2: Thermal and General Properties of PLA Formulations
| Material Formulation | Heat Deflection Temperature (HDT) | Melting Point (°C) | Key Characteristics for Load-Bearing | Reference |
|---|---|---|---|---|
| Neat PLA | 53 - 56 °C | 150 - 162 | Brittle; poor heat resistance; degrades under load at low elevated temperatures. | [81] [80] |
| Cross-linked PLA (with TAIC) | Much improved heat stability; rubbery, soft, and stable at higher temperatures, even over Tm. | Not Specified | Improved heat stability and mechanical properties at high temperatures; degradation considerably retarded. | [1] |
| Stereo-complex PLA (SC-PLA) | Not Specified | > 200 °C | Significantly improved heat resistance; melting point elevated. | [27] |
Analysis of Comparative Data
- Neat PLA: The data confirms that neat PLA, while possessing moderate tensile strength, has significant limitations. Its brittleness (low elongation at break) is a critical failure point for dynamic or impact loads [80]. Furthermore, its low HDT means it cannot be used in environments where temperatures exceed ~60°C, as it will deform under minimal load [81].
- Fiber-Reinforced PLA: The incorporation of short carbon fibers (CF) leads to a dramatic improvement in both tensile and flexural performance. The documented 47.1% increase in tensile strength and 230.95% increase in flexural stiffness transforms PLA from a prototyping material into a candidate for functional, lightweight structural components [82]. The fibers carry a significant portion of the applied load, resulting in higher strength and stiffness.
- Cross-Linked PLA: Cross-linking addresses different limitations. Instead of primarily increasing strength and stiffness, it enhances thermal stability and creep resistance. The ability of cross-linked PLA to remain "rubbery, soft, and stable at higher temperatures, even over Tm" is a transformative property for applications requiring shape retention under thermal stress [1]. Furthermore, the cross-linked network retards the hydrolysis of ester linkages, thereby slowing down the degradation process, which is crucial for long-term implants [1].
Cross-Linking Methodologies and Experimental Protocols
Cross-linking creates a three-dimensional network within the PLA matrix, fundamentally altering its properties. The following diagram illustrates the primary methodological pathways for achieving cross-linking in PLA, detailed in the experimental protocols below.
Diagram Title: Pathways for Cross-Linking PLA.
High-Energy Irradiation Cross-Linking
This method utilizes ionizing radiation to generate radicals on polymer chains, inducing cross-linking.
- Materials: PLA polymer resin (e.g., PLLA or a blend of PLLA/PDLA), polyfunctional monomer (e.g., Triallyl isocyanurate - TAIC, Trimethylolpropane trimethacrylate - TMPTMA) [1].
- Protocol:
- Preparation: Dry PLA pellets and polyfunctional monomers thoroughly to remove moisture.
- Mixing: Blend PLA with the cross-linking co-agent (e.g., ~3% TAIC by weight) using a twin-screw extruder or similar mixer to ensure a homogeneous distribution [1].
- Processing: The blended material is then typically compression molded or extruded into sheets or films of the desired thickness for testing.
- Irradiation: Expose the samples to a controlled dose of high-energy radiation (e.g., electron beam at 30–50 kGy) [1]. The dose rate and temperature during irradiation must be controlled, as they influence the cross-linking density and competing chain scission reactions.
- Post-processing: The cross-linked material is then ready for characterization. Gel content analysis is commonly used to determine the degree of cross-linking.
Chemical Cross-Linking with Isocyanates
This method uses chemical agents to form covalent bonds between polymer chains and/or reinforcing fibers.
- Materials: PLA polymer (PLLA and PDLA to form stereo-complex), bamboo fiber (BF) or other reinforcements, polyaryl polymethylene isocyanate (PAPI) as a cross-linker, acetone as a solvent [27].
- Protocol:
- Material Drying: Dry PLLA, PDLA, and bamboo fiber (e.g., at 80-100°C for 5 hours) [27].
- Cross-linker Dispersion: Disperse the PAPI cross-linker in acetone by stirring to create a uniform solution [27].
- Surface Coating: Spray the PAPI-acetone solution onto the surface of the PLLA/PDLA pellets and dry to remove the solvent, ensuring the cross-linker coats the polymer.
- Compounding and Molding: Mix the coated pellets with bamboo fiber according to the desired formulation (e.g., 15% BF). Process the mixture using a twin-screw extruder (e.g., at 200–220°C) and subsequently injection mold into test specimens [27].
The Scientist's Toolkit: Key Research Reagents and Materials
Table 3: Essential Materials for PLA Cross-Linking and Reinforcement Research
| Reagent/Material | Function | Example in Context |
|---|---|---|
| Triallyl Isocyanurate (TAIC) | A polyfunctional monomer that enhances the efficiency of radiation-induced cross-linking by providing multiple reactive sites. | Used at ~3% concentration during electron-beam irradiation to promote network formation instead of chain scission [1]. |
| Polyaryl Polymethylene Isocyanate (PAPI) | A cross-linking agent that forms urethane linkages with hydroxyl and carboxyl groups, creating bridges between PLA chains and natural fibers. | Used to form cross-linked structures between stereo-complex PLA and bamboo fiber, improving compatibility and mechanical properties [27]. |
| PLLA / PDLA | The two stereoisomers of PLA. Blending them promotes the formation of stereo-complex crystals (SC-PLA). | SC-PLA has a higher melting point (>200°C) and improved heat resistance, forming a base for high-performance composites [27]. |
| Short Carbon Fibers (CF) | A discontinuous reinforcement filler that significantly increases the stiffness and strength of the polymer matrix. | Added to PLA to create composites for Fused Filament Fabrication (FFF), dramatically improving tensile and flexural properties [82] [81]. |
| Bamboo Fiber (BF) | A low-cost, renewable biomass filler used to reduce material cost and environmental impact; requires compatibilization. | Added to SC-PLA composites; when cross-linked with PAPI, tensile strength increased by 85.5% compared to unmodified composite [27]. |
Degradation and Crystallization Behavior
The introduction of cross-links has a profound impact on two phenomena central to the broader thesis: crystallization and degradation.
Effect on Crystallization: Cross-linking restricts the mobility of polymer chains, which can initially hinder the formation of large, ordered crystalline regions. However, it can also act as a nucleating site. In contrast, mechanical recycling (chain scission) leads to shorter polymer chains that can reorganize more easily, causing a significant increase in crystallinity (e.g., from 6.9% to 39.5% after six extrusion cycles) [83]. This increased crystallinity, while improving stiffness, also embrittles the material.
Effect on Degradation: The cross-linked network presents a barrier to water penetration and slows the hydrolysis of ester bonds in the polymer backbone. This retards the degradation rate of PLA, which is desirable for applications requiring longer service life [1]. Conversely, chain scission during recycling drastically reduces molecular weight, which would normally accelerate degradation, but the associated increase in crystallinity can make the crystalline regions more resistant to hydrolysis, potentially leading to a slower overall biodegradation rate [83]. This inverse relationship between the outcomes of cross-linking and chain scission underlines the critical importance of molecular architecture in controlling material lifetime.
In the pursuit of sustainable plastics, poly(lactic acid) (PLA) has emerged as a leading bio-based and biodegradable alternative to conventional petrochemical-based polymers. However, its broader application is often limited by inherent shortcomings, including relatively low heat resistance, brittleness, and slow degradation rate under ambient conditions [84] [85]. These properties are intrinsically linked to the polymer's molecular structure and crystallization behavior. Cross-linking has been established as a powerful strategy to engineer polymer properties, creating a three-dimensional network that profoundly impacts material performance [86]. This guide provides a comparative analysis of cross-linking strategies for PLA, evaluating their effects on thermal properties, dimensional stability, crystallization, and degradation. We focus on presenting objective experimental data to help researchers select and optimize cross-linking methods for specific applications.
Comparative Analysis of Cross-Linking Approaches and Performance
Cross-linking modifies PLA properties by forming covalent bonds between polymer chains, creating a network that restricts chain mobility. This restriction directly enhances heat resistance and dimensional stability but also influences crystallinity and degradation profiles. The following table summarizes key performance metrics for different cross-linking systems as reported in recent studies.
Table 1: Performance Comparison of Cross-Linked PLA Systems
| Cross-Linking System / Material | Key Performance Metrics | Effect on Crystallinity | Degradation Profile |
|---|---|---|---|
| γ-ray Cross-linked PLLA/TAIC [22] | Shape recovery ratio: 99.5% (10 wt% TAIC); Maintains 97.9% after 3 cycles. Recovers from 800% strain. | Crosslinking points suppress cold crystallization and prevent irreversible chain slippage. | Not the primary focus of the study. |
| PLA/Thionoester Copolymer [71] | Introduction of weak thionoester linkages into the backbone. | Thermal properties (TGA/DSC) were assessed, though specific crystallinity data not provided. | Selective degradation in response to specific stimuli (e.g., amines); Enhanced aqueous degradation. |
| PLA/Thermoplastic Alginate (TPA) [84] | Tensile strength and thermal stability decreased with TPA addition. | TPA acted as a nucleating agent, enhancing the crystallinity of PLA. | Soil burial degradation rate significantly increased with TPA content. |
| PLA/Carbon Quantum Dots (CQDs) [85] | Simultaneous improvement in tensile strength, modulus, and thermal stability. | CQDs acted as nucleating agents, facilitating crystallization and increasing crystallization temperature. | Accelerated degradation under hydrolysis, PBS immersion, and soil burial conditions. |
Experimental Protocols for Key Cross-Linking Methodologies
γ-Ray Irradiation Cross-Linking with TAIC
This method uses high-energy radiation to create free radicals, leading to covalent bonds between PLA chains and the cross-linking agent triallyl isocyanurate (TAIC).
- Materials Preparation: PLLA (e.g., NatureWorks 3001D) is dried at 80 °C for 12 hours. It is then melt-blended with TAIC (1-10 wt%) at 190 °C for 10 minutes using an internal mixer. The homogeneous blend is hot-pressed into films (e.g., 300 μm thickness) at 190 °C under 10 MPa pressure [22].
- Irradiation Process: The prepared specimens are vacuum-sealed and irradiated using a ^60^Co γ-ray source at a specified dose (e.g., 30 kGy) at room temperature [22].
- Characterization:
- Shape Memory Testing: The shape recovery ratio is measured via a uniaxial stretching experiment. A specimen is stretched to a specific strain (e.g., 100% or 800%) at 80 °C (above Tg), cooled to 25 °C to fix the temporary shape, and then reheated to measure recovery to the permanent shape [22].
- Gel Content: The cross-linking density is quantified by measuring the gel fraction, which is the insoluble part after soaking the cross-linked material in a solvent like chloroform for 48 hours [22].
Incorporation of Degradable Additives: Carbon Quantum Dots
This approach enhances properties by adding nano-sized fillers that facilitate crystallization and potentially accelerate degradation, without forming permanent covalent networks.
- CQDs Synthesis: Carbon quantum dots are synthesized from biomass (e.g., wheat straw) via a hydrothermal method. The biomass is processed and heated in a sealed reactor at elevated temperatures (e.g., 200 °C for several hours) to form CQDs, which are then purified [85].
- Composite Fabrication: PLA/CQDs composite films are prepared using a solution casting method. PLA is dissolved in a suitable solvent (e.g., trichloromethane), and a specific weight percentage of CQDs (e.g., 0.4 wt%) is dispersed into the solution. The mixture is cast onto a plate and the solvent is allowed to evaporate, forming a film [85].
- Characterization:
- Thermal Analysis: Thermogravimetric Analysis (TGA) assesses thermal stability by measuring weight loss as a function of temperature. Differential Scanning Calorimetry (DSC) analyzes melting and crystallization behavior [85].
- Mechanical Testing: Tensile strength, Young's modulus, and elongation at break are determined using a universal testing machine [85].
- Degradation Studies: Films are subjected to hydrolysis (in NaOH solution), phosphate-buffered saline (PBS) immersion, and soil burial tests, with weight loss measured over time [85].
Pathways and Workflows in Cross-Linked PLA Systems
The following diagram illustrates the experimental workflow for creating and evaluating γ-ray cross-linked PLA, a method that provides exceptional shape memory properties.
Diagram 1: Workflow for γ-ray cross-linked PLA/TAIC preparation and characterization.
The Scientist's Toolkit: Key Research Reagents and Materials
Successful experimentation in PLA cross-linking requires specific reagents and equipment. The following table details essential items and their functions based on the cited protocols.
Table 2: Essential Research Reagents and Materials for Cross-Linked PLA Studies
| Material / Reagent | Function in Research | Example from Literature |
|---|---|---|
| Poly(L-lactic acid) (PLLA) | The primary polymer matrix for cross-linking and modification. | NatureWorks 3251D (injection grade) or 3001D [84] [85]. |
| Triallyl Isocyanurate (TAIC) | A cross-linking agent that forms covalent networks upon irradiation. | Used at 1-10 wt% in PLLA for γ-ray cross-linking [22]. |
| Di-tert-butyl peroxide (DTBP) | A peroxide initiator that generates free radicals for cross-linking reactions. | Used as a primary cross-linker in HDPE systems; applicable in PLA modification [87]. |
| Carbon Quantum Dots (CQDs) | Nano-sized bio-based filler that enhances crystallization and accelerates degradation. | Synthesized from wheat straw and added at 0.4 wt% to PLA [85]. |
| Thermoplastic Alginate (TPA) | A bio-based polymer additive that increases hydrophilicity and biodegradation rate. | Blended with PLA at 5-30 wt% to form biocomposites [84]. |
| Tin(II) 2-ethylhexanoate (Sn(Oct)â‚‚) | A catalyst for ring-opening polymerization, e.g., for synthesizing PLA copolymers. | Used in the ring-opening copolymerization of lactide and thiocarbonyl lactide [71]. |
| γ-ray Irradiation Source (^60^Co) | Provides high-energy photons to initiate radical-based cross-linking in solid state. | Used to cross-link PLLA/TAIC blends with a dose of 30 kGy [22]. |
The cross-linking strategy selected for PLA directly dictates the final property profile of the material. Radiation cross-linking with agents like TAIC creates robust covalent networks, leading to exceptional dimensional stability and shape memory effects, making it suitable for applications requiring high elastic recovery. In contrast, the incorporation of degradable additives or fillers like CQDs or TPA offers a different pathway, primarily enhancing crystallization and accelerating the material's environmental degradation. This approach is valuable for single-use applications where end-of-life considerations are paramount. The choice between creating a permanent network or a degradable composite must be guided by the specific performance requirements and the intended lifecycle of the final product.
The development of controlled release systems is a pivotal area of research in modern drug delivery, particularly for chemotherapeutic agents where precise dosing and sustained release are critical for therapeutic efficacy and patient compliance. Among the various polymeric carriers, poly(lactic-co-glycolic acid) (PLGA) has emerged as a frontrunner due to its excellent biocompatibility and tunable degradation properties. This guide provides a comprehensive comparative analysis of cross-linked PLGA carriers, examining their controlled release profiles and tissue response against non-cross-linked alternatives and other biodegradable polyesters like PCL and PLA. Framed within a broader thesis on cross-link effects on crystallization and degradation in poly(lactic acid) research, this article synthesizes current experimental data to guide researchers and drug development professionals in selecting and optimizing PLGA-based systems for specific biomedical applications. The fundamental properties of these polymers provide the foundation for understanding how cross-linking modifies their behavior, as summarized in Table 1.
Table 1: Fundamental Properties of Key Biodegradable Polyesters
| Property | PCL | PLA | PLGA | Cross-linked PLGA |
|---|---|---|---|---|
| Chemical Composition | Semi-crystalline aliphatic polyester from ε-caprolactone [70] | Aliphatic polyester from L-lactide and D-lactide isomers [70] | Copolymer of lactic acid (LA) and glycolic acid (GA) [70] | Covalently cross-linked PLGA network [88] [89] |
| Crystallinity | High (20-33%) [70] | Varies with D/L isomer ratio [70] | Amorphous to semi-crystalline [70] | Amorphous, cross-link inhibited [88] |
| Glass Transition Temp. (T_g) | ≈ -60°C [70] | ≈ 60°C [70] | 40-60°C [70] | Increased T_g post-cross-linking [88] |
| Degradation Timeline | Very slow (years) [70] | Several months [70] | Weeks to months (LA:GA ratio-dependent) [70] | Significantly retarded [88] [90] |
| Primary Degradation Mode | Hydrolysis (slow, enzymatic) [70] | Non-enzymatic hydrolysis [70] | Non-enzymatic hydrolysis [70] | Hydrolysis of ester segments in backbone [89] |
Comparative Analysis of Cross-Linked vs. Non-Cross-Linked PLGA Carriers
Degradation Kinetics and Drug Release Profiles
The introduction of cross-links into the PLGA matrix fundamentally alters its hydrolytic degradation behavior and, consequently, the release kinetics of encapsulated active compounds.
- Retarded Hydrolysis: Cross-linked PLGA exhibits a pronounced retardation in polymer hydrolysis compared to its non-cross-linked counterparts. Studies have shown that this is due to the three-dimensional network that limits water penetration and reduces the rate of ester bond cleavage [88]. The degradation rate can be finely tuned by the cross-linking parameters, such as radiation dose and the concentration of cross-linking agents like pentaerythritol tetraacrylate (PTTA) [88].
- Impact of Terminal Groups: Recent research highlights that modifying PLGA's terminal groups is another powerful strategy to control degradation. PLGA microspheres with terminal groups featuring longer carbon chains (e.g., dodecyl, hexadecyl) demonstrate a slower degradation rate than those with standard carboxyl termini. This occurs because the hydrophobic alkyl chains impede water access to the polymer matrix, thereby delaying the onset of bulk erosion [90].
- Consequences for Drug Release: The modified degradation profile directly translates to a more sustained and controlled drug release. For a drug like cyproterone acetate, release from PLGA microspheres with various terminal groups could be sustained for up to two months, achieving nearly 100% recovery [90]. Cross-linking minimizes the characteristic initial "burst release," leading to a release profile that better approximates zero-order kinetics, which is highly desirable for maintaining stable plasma drug concentrations [88].
Mechanical and Thermal Properties
Cross-linking significantly enhances the structural integrity of PLGA carriers, which is crucial for applications requiring mechanical stability.
- Enhanced Mechanical Performance: Electron beam cross-linking of PLGA and PLLA with PTTA has been shown to increase tensile strength and Young's modulus. The optimal cross-linking dose was found to be 3 Mrad, yielding the highest gel fraction and the most significant enhancement of mechanical properties for both polymers [88].
- Improved Thermal Stability: The same cross-linking process increases the glass transition temperature (Tg) of the polymers. This elevation in Tg indicates greater thermal stability and reduced chain mobility at physiological temperatures, contributing to the overall structural robustness of the carrier [88].
- Sequenced Oligomer Cross-Linking: Innovative approaches using precisely sequenced oligolactoglycolic acid dimethacrylates (OLGADMAs) to create cross-linked NPs result in "heightened stability." These NPs exhibit minimal pH changes over five weeks, unlike conventional PLGA, which undergoes acidic degradation, causing autocatalytic breakdown. This enhanced stability is attributed to the cross-linked structure and the absence of acidic end-groups [89].
Biocompatibility and Tissue Response
The biological performance of a drug delivery system is paramount. Cross-linked PLGA carriers have demonstrated a favorable safety profile in pre-clinical studies.
- Minimal Cytotoxicity: Cross-linked nanoparticles derived from OLGADMAs show minimal cytotoxicity in vitro, supporting their excellent biocompatibility [89]. Furthermore, in vivo studies on drug-loaded PLGA-PEG microspheres for triple-negative breast cancer treatment showed that the formulations did not cause any noticeable toxicity and significantly extended the survival of treated mice post-tumor resection [91].
- Stealth Properties and Targeting: PEGylation—the conjugation of polyethylene glycol (PEG)—creates a hydrophilic corona on PLGA nanoparticles. This corona reduces opsonization and recognition by the immune system, prolonging systemic circulation time and increasing the likelihood of the carrier reaching its target site [92] [93]. This surface can be further functionalized with targeting ligands (e.g., LHRH) for selective delivery to specific cells via ligand-receptor interactions, enhancing cellular uptake and reducing off-target effects [91] [93].
Experimental Protocols for Key Analyses
Protocol: Electron Beam Radiation Cross-Linking
This protocol describes a standard method for cross-linking PLGA films using electron beam radiation [88].
- Film Preparation: Dissolve PLGA polymer and a poly-functional monomer (PFM) cross-linking agent (e.g., Pentaerythritol tetraacrylate, PTTA) in a volatile organic solvent such as dichloromethane. Cast the solution into a mold and allow the solvent to evaporate fully to form a thin film.
- Irradiation: Subject the dried films to electron beam radiation at a predetermined dose (e.g., 1-5 Mrad) under an inert atmosphere to minimize oxidative degradation.
- Post-Irradiation Analysis:
- Gel Fraction Measurement: Extract the irradiated films in a suitable solvent (e.g., dichloromethane) for 24 hours to remove sol (non-cross-linked polymer). Dry the remaining gel and calculate the gel fraction as (weight of dried gel / initial weight) × 100%.
- Mechanical Testing: Characterize the tensile strength and elastic modulus of the cross-linked films using a universal testing machine.
- Thermal Analysis: Determine the glass transition temperature (T_g) using Differential Scanning Calorimetry (DSC).
Protocol: Synthesis of Sequenced Cross-Linked NPs
This protocol outlines the synthesis of highly stable, cross-linked PLGA-inspired nanoparticles via a one-pot nanopolymerization technique [89].
- Monomer Synthesis: Synthesize precisely sequenced oligolactoglycolic acid dimethacrylates (OLGADMAs) from lactic and glycolic acid derivatives via a multi-step procedure involving carbodiimide chemistry and methacrylation.
- Nanoprecipitation-Polymerization:
- Dissolve the synthesized OLGADMA monomer and a hydrophobic drug (e.g., Dexamethasone) in a water-miscible organic solvent (e.g., acetone).
- Rapidly add this solution into an aqueous phase containing a radical initiator (e.g., VA-044) under vigorous stirring.
- Allow the reaction to proceed for 24 hours at room temperature to facilitate simultaneous nanoparticle formation and radical polymerization/cross-linking of the methacrylate groups.
- Purification and Characterization: Purify the resulting nanoparticle suspension via dialysis or centrifugation. Characterize particle size and zeta potential using Dynamic Light Scattering (DLS). Determine drug encapsulation efficiency using HPLC.
Protocol: In Vitro Drug Release and Degradation Kinetics
This is a general protocol for assessing the performance of cross-linked PLGA carriers [91] [90].
- Release Study Setup: Place a precise amount of drug-loaded microspheres or nanoparticles into a release medium (e.g., phosphate-buffered saline, PBS) at a specific temperature (e.g., 37°C to mimic human body temperature).
- Sampling: At predetermined time intervals, centrifuge the samples to separate the released drug from the particles. Withdraw a aliquot of the supernatant for analysis and replace it with fresh pre-warmed medium to maintain sink conditions.
- Analysis:
- Drug Quantification: Analyze the drug concentration in the supernatant using a validated method, such as UV-Vis spectroscopy or HPLC.
- Polymer Degradation: In parallel, isolate a separate set of particles at different time points. Analyze their molecular weight loss using Gel Permeation Chromatography (GPC) and monitor morphological changes using Scanning Electron Microscopy (SEM).
Figure 1: Experimental workflows for cross-linking (yellow), nanoparticle synthesis (green), and performance analysis (red).
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Reagents and Materials for PLGA Cross-Linking and Analysis
| Item Name | Function / Application | Key Characteristic / Rationale |
|---|---|---|
| Pentaerythritol Tetraacrylate (PTTA) | Tetra-functional cross-linking monomer for radiation-induced cross-linking [88]. | Multi-functional groups increase cross-linking efficiency and enhance mechanical properties [88]. |
| Oligolactoglycolic Acid Dimethacrylates (OLGADMAs) | Precisely sequenced building blocks for novel cross-linked NPs [89]. | Allows for control over polymer sequence and structure, leading to highly stable nanoparticles [89]. |
| Polyethylene Glycol (PEG) | PEGylation agent for surface modification of PLGA carriers [92] [93]. | Imparts "stealth" properties, reducing immune clearance and prolonging circulation half-life [93]. |
| Luteinizing Hormone-Releasing Hormone (LHRH) Ligand | Targeting ligand for functionalizing nanoparticle surface [91]. | Binds to receptors overexpressed on cancer cells (e.g., TNBC), enabling active targeting [91]. |
| VA-044 Initiator | Water-soluble azo initiator for radical polymerization [89]. | Used in the one-pot nanopolymerization of OLGADMAs to form cross-linked NPs [89]. |
Cross-linked PLGA carriers represent a significant advancement over traditional non-cross-linked systems and other biodegradable polyesters like PCL and PLA. The strategic introduction of cross-links, whether through radiation chemistry, terminal group modification, or advanced sequenced oligomer polymerization, provides unparalleled control over degradation kinetics, drug release profiles, and mechanical integrity. These systems successfully address critical challenges such as initial burst release, acidic degradation, and unpredictable erosion, paving the way for more reliable and effective long-acting injectable formulations. While the field has made remarkable progress, the adoption of machine learning for synthesis optimization [94] and the development of novel, precisely sequenced monomers [89] point toward a future of intelligent, tailored drug delivery systems. Cross-linked PLGA platforms hold immense potential for clinical translation, promising to enhance the treatment of chronic diseases, including cancer, by enabling precision medicine and improving patient outcomes through sustained and targeted therapy.
Polylactic Acid (PLA) represents a leading bio-based polymer in the shift toward sustainable materials. Cross-linking modifies its properties, enhancing thermal and mechanical performance for broader applications. This guide compares the environmental profile of cross-linked PLA against conventional PLA and petrochemical alternatives, using Life Cycle Assessment (LCA) to evaluate the carbon footprint and other environmental impacts. Data indicates that while cross-linking can improve material performance and longevity, it introduces trade-offs in energy consumption and end-of-life biodegradability that must be carefully managed within a circular economy framework.
The global plastics crisis has intensified the search for sustainable alternatives to petroleum-based polymers. PLA, a biodegradable and bio-based aliphatic polyester derived from renewable resources like corn or sugarcane, has emerged as a prominent candidate [95] [1]. Its production is projected to grow significantly, with the market expected to expand from USD 968.74 million in 2024 to approximately USD 3,864.79 million by 2034 [96]. However, widespread adoption of PLA is hampered by inherent limitations, including poor heat resistance (heat resistance temperature approximately 60°C) and inherent brittleness [27] [56].
Cross-linking is a key modification strategy to overcome these drawbacks. It involves creating a network structure within the polymer, leading to enhanced thermal stability, mechanical strength, and toughness [1] [97]. These improvements expand PLA's applicability into demanding sectors such as heat-resistant packaging, automotive components, and durable goods. However, this modification also influences the environmental profile of PLA products across their entire life cycle—from feedstock acquisition to end-of-life (EoL) disposal. This guide provides a comparative LCA of cross-linked PLA products, detailing their environmental impact and carbon footprint in relation to unmodified PLA and common petroleum-based polymers.
Life Cycle Assessment (LCA) of PLA: A Primer
LCA is a standardized methodology for evaluating the environmental impacts associated with all stages of a product's life, from "cradle to grave" [95]. For PLA, the lifecycle is typically divided into four main stages [95]:
- Feedstock Collection and Conversion: Cultivation of biomass (e.g., corn, sugarcane), fermentation into lactic acid, and polymerization into PLA.
- Processing: Manufacturing of PLA into final products (e.g., via extrusion, injection molding).
- Use: The operational life of the product.
- End-of-Life (EoL): Disposal or recovery via recycling, composting, incineration, or landfilling.
The system boundary for PLA production includes inputs of energy, materials, and outputs such as greenhouse gas (GHG) emissions and other pollutants [95]. A critical finding from LCA studies is that the conversion process is the most energy-intensive stage, with natural gas and electricity accounting for 65% and 22% of the total energy used, respectively, in the production of one kilogram of PLA from corn [95]. This highlights that despite its bio-based origins, PLA's environmental footprint is still closely tied to fossil fuels used for process energy.
Environmental Impact of Cross-Linked vs. Conventional PLA
Cross-linking alters the properties of PLA, which in turn affects its environmental impact throughout its life cycle. The following table summarizes the key differences in properties and environmental implications.
Table 1: Comparison of Conventional PLA and Cross-Linked PLA
| Aspect | Conventional PLA | Cross-Linked PLA |
|---|---|---|
| Primary Feedstock | Corn, sugarcane [95] | Corn, sugarcane (with cross-linking additives) [97] |
| Key Mechanical/Thermal Properties | Tensile strength: 15.5-150 MPa; Heat resistance: ~60°C [95] [27] | Enhanced tensile strength and heat stability above Tg/Tm [1] [97] |
| Typical EoL Biodegradation | Biodegradable under industrial composting conditions [95] | Degradation considerably retarded; slower hydrolysis [1] |
| Major LCA Hotspot | Energy for polymer conversion [95] | Energy for polymer conversion + embedded energy of cross-linking agents/processes [1] |
| Carbon Footprint (General) | Lower GHG emissions than many fossil-based plastics [95] [98] | Potential increase due to added processing, but avoided production from extended lifespan [27] |
The incorporation of cross-linking agents, while improving performance, adds a layer of complexity to the LCA. For instance, the production of agents like triallyl isocyanurate (TAIC) or the use of irradiation processes carries its own environmental burden. Furthermore, cross-linking creates a network structure that retards biodegradation and can complicate mechanical recycling [1]. This means that while cross-linked PLA products may offer superior durability and potentially replace more carbon-intensive materials, their EoL phase requires careful management to avoid negative environmental consequences.
Case Study: LCA of Cross-Linked Bamboo Fiber/PLA Composites
Research on cross-linked stereo-complex PLA (SC-PLA) with bamboo fiber (BF) provides quantitative LCA data. A study found that adding 15% BF to a SC-PLA composite and modifying it with polyaryl polymethylene isocyanate (PAPI) resulted in an 85.5% increase in tensile strength [27]. From an LCA perspective, the addition of BF itself was beneficial:
- CO2 emissions decreased by approximately 11.7% compared to the SC-PLA composite without BF.
- Significant reductions were also observed in freshwater, marine, and land ecotoxicity [27].
This demonstrates that using bio-based fillers can mitigate the environmental impact of the composite, even after accounting for the cross-linking process. The cross-linking agent, in this case PAPI, was crucial for ensuring strong interfacial adhesion between the BF and the PLA matrix, leading to a high-performance material with a comparatively improved environmental profile.
Comparative LCA: Cross-Linked PLA vs. Petrochemical Polymers
To contextualize the environmental performance of cross-linked PLA, it is essential to compare it with conventional petrochemical polymers it aims to replace, such as Polypropylene (PP) and Polyethylene Terephthalate (PET).
Table 2: LCA Comparison of Cross-Linked PLA with Petrochemical Polymers (per single-use cup functional unit) [98]
| Environmental Impact Category | Cross-Linked PLA | PET | PP | Interpretation |
|---|---|---|---|---|
| Climate Change (GHG Emissions) | Lower than PET | Higher than PLA | Lowest | PLA offers climate benefits over PET but may not outperform PP. |
| Fossil Resource Use | Lowest | Higher | Higher | PLA is superior due to its bio-based feedstock. |
| Photochemical Ozone Formation | Higher | Lower | Lower | PLA production and land use changes can lead to higher smog formation. |
| Acidification | Higher | Lower | Lower | Agricultural practices for biomass growth contribute to acidification. |
| Terrestrial Eutrophication | Higher | Lower | Lower | Fertilizer use in corn/sugarcane cultivation is a primary contributor. |
A pivotal LCA study of single-use cups concluded that conventional PLA cups outperform PET cups in terms of climate change and fossil resource use but do not necessarily outperform PP cups from an overall environmental perspective [98]. The impact of land use change was significant for climate change and photochemical ozone formation in PLA [98]. For cross-linked PLA, this comparison forms a baseline; the enhanced properties of cross-linked PLA could justify its use in more durable applications, where its longer lifespan and potential to replace less sustainable materials could improve its relative environmental performance over single-use scenarios.
Experimental Protocols for Cross-Linking and Analysis
Common Cross-Linking Methodologies
Researchers employ several methods to introduce cross-links into PLA, each with specific protocols and implications.
Chemical Cross-Linking:
- Protocol: PLA is melt-mixed with a cross-linking agent (e.g., Triallyl Isocyanurate - TAIC) and an initiator (e.g., Dicumyl Peroxide - DCP) in an internal mixer (e.g., at 180°C, 50 rpm for 10 min). The resulting material is then compression-molded into sheets for testing [97].
- Mechanism: The peroxide initiator generates free radicals upon heating, which abstract hydrogen from the PLA backbone. The TAIC radicals then form covalent bonds between multiple PLA chains, creating a network [97].
High-Energy Irradiation Cross-Linking:
- Protocol: PLA samples, often with the addition of polyfunctional monomers (e.g., TAIC, TMPTMA), are exposed to gamma rays or an electron beam from a Cobalt-60 source at specified doses (e.g., 30–150 kGy) in air at room temperature [1] [99].
- Mechanism: Ionizing radiation generates radicals directly on the polymer chains. In the presence of cross-linking co-agents, these radicals preferentially lead to the formation of a three-dimensional network rather than chain scission [1].
Reactive Compatibilization/Cross-Linking:
- Protocol: For PLA blends (e.g., with PBAT or P(3HB-co-4HB)), a compatibilizer like epoxidized soybean oil (ESBO) is added during twin-screw extrusion (e.g., at 200–220°C). The epoxy groups of ESBO can react with the terminal carboxyl or hydroxyl groups of the polyesters, forming cross-linked structures at the interface [56].
Key Characterization Techniques
- Gel Content: Measures the fraction of insoluble polymer after solvent extraction, directly indicating the degree of cross-linking [97].
- Thermal Analysis (DSC & TGA): Differential Scanning Calorimetry (DSC) assesses changes in glass transition (Tg), melting temperature (Tm), and crystallinity. Thermogravimetric Analysis (TGA) evaluates thermal stability by measuring weight loss against temperature [97] [99].
- Mechanical Testing: Tensile and flexural tests per ASTM standards (e.g., D638, D790) quantify improvements in strength, modulus, and elongation at break [27] [97].
- Rheological Analysis: Reveals changes in melt viscosity and viscoelastic behavior, which typically increase with cross-linking density [99].
The following workflow diagram illustrates the logical relationship between the cross-linking process, the resulting material changes, and the subsequent LCA evaluation.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for PLA Cross-Linking Research
| Reagent/Equipment | Function in Research | Example Use Case |
|---|---|---|
| Triallyl Isocyanurate (TAIC) | Polyfunctional cross-linking agent; its double bonds form bridges between PLA chains. | Used in chemical (with DCP) [97] and irradiation-induced [1] cross-linking. |
| Dicumyl Peroxide (DCP) | Thermal initiator; decomposes to generate free radicals that kick-start the cross-linking reaction. | Initiating chemical cross-linking of PLA with TAIC in melt-mixing [97]. |
| Epoxidized Soybean Oil (ESBO) | Bio-based compatibilizer and cross-linker; epoxy groups react with PLA end groups. | Improving toughness and compatibility in PLA/P(3HB-co-4HB) blends [56]. |
| Gamma Irradiator (Co-60) | High-energy radiation source; induces cross-linking (often with co-agents) or chain scission. | Cross-linking PLA/PBAT blends to enhance compatibility [99]. |
| Polyaryl Polymethylene Isocyanate (PAPI) | Cross-linking agent; forms urethane linkages with hydroxyl groups, reinforcing composites. | Strengthening interface in bamboo fiber/PLA composites [27]. |
The LCA of cross-linked PLA products reveals a complex interplay between performance enhancement and environmental impact. The primary environmental burden for both conventional and cross-linked PLA remains the energy-intensive conversion process. Cross-linking can improve material properties, enabling applications that may displace more carbon-intensive materials, but it can also hinder biodegradability and complicate recycling.
Future development should focus on optimizing this trade-off. Key areas include:
- Developing bio-based and less toxic cross-linking agents.
- Utilizing renewable energy in PLA production and processing.
- Designing cross-linked PLA materials for specific, durable applications where their longer service life maximizes the environmental benefit.
- Exploring cleavable cross-links that maintain performance during use but allow for degradation or recycling at EoL.
As the market for PLA continues to grow [96], informed by robust LCA data, the evolution of cross-linked PLA products will play a significant role in the transition to a more sustainable and circular materials economy.
Conclusion
This analysis demonstrates that cross-linking serves as a powerful strategy for fundamentally reshaping the property profile of PLA, enabling precise control over both crystallization behavior and degradation kinetics. The establishment of cross-linked networks enhances melt strength, thermal resistance, and mechanical durability while providing a mechanism to program degradation timelines from weeks to months. Future research should focus on developing more biocompatible cross-linking chemistries, multi-stimuli responsive networks, and advanced manufacturing techniques that preserve cross-link integrity during processing. The integration of computational modeling with experimental validation will further accelerate the design of application-specific PLA materials, particularly for long-acting drug delivery implants and high-performance biodegradable medical devices. As the field advances, cross-linked PLA systems are poised to bridge critical performance gaps between conventional bioplastics and engineering-grade polymers, unlocking new frontiers in sustainable materials and precision medicine.
References
- 1. Crosslinking of Polylactide by High Energy Irradiation and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7660633/]
- 2. Comparison study of hydrolytic degradation behaviors ... [https://www.sciencedirect.com/science/article/abs/pii/S0141391017303841]
- 3. In-situ cross-linking strategy for achieving excellent impact ... [https://www.sciencedirect.com/science/article/abs/pii/S0141813025012188]
- 4. The reactive compatibilization of PLA/PP blends and ... [https://pubs.rsc.org/en/content/articlelanding/2021/ce/d0ce01445a]
- 5. Effect of poly(lactic acid) crystallization on its mechanical ... [https://www.sciencedirect.com/science/article/abs/pii/S0032386120311058]
- 6. Accelerating Reactive Compatibilization of PE/PLA Blends ... [https://pubmed.ncbi.nlm.nih.gov/35596396/]
- 7. Effects of crosslink density on hydrolytic degradation ... [https://www.sciencedirect.com/science/article/abs/pii/S014139101200095X]
- 8. Crystallization study and comparative in vitro-in vivo ... [https://pubmed.ncbi.nlm.nih.gov/22072906/]
- 9. The Effects of Nucleating Agents and Processing on ... [https://www.mdpi.com/2072-666X/15/6/776]
- 10. Comparison of the efficiency of the most effective ... [https://link.springer.com/article/10.1007/s10973-021-11145-y]
- 11. Crystallization and degradation behaviour of multiblock ... [https://link.springer.com/article/10.1557/s43579-021-00107-y]
- 12. Effect of Bio-Based Plasticizer on Crystallization Kinetics ... [https://pubmed.ncbi.nlm.nih.gov/41228696/]
- 13. Crystallization of Polylactic Acid with Organic Nucleating ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10857276/]
- 14. Effect of Bio-Based Plasticizer on Crystallization Kinetics ... [https://www.mdpi.com/2073-4360/17/21/2935]
- 15. Sustainable Plastics: Effect of Bio-Based Plasticizer on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12608812/]
- 16. Hydrolytic degradation and lifetime prediction of poly(lactic ... [https://www.sciencedirect.com/science/article/abs/pii/S0142941819312577]
- 17. A Perspective on Polylactic Acid-Based Polymers Use for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6797553/]
- 18. Analysis of the hydrolysis behavior of poly(lactic acid) (PLA ... [https://www.sciencedirect.com/science/article/abs/pii/S0141391025001028]
- 19. Controlled and Accelerated Hydrolysis of Polylactide (PLA) ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9573666/]
- 20. Hydrolytic degradation mechanism of modified polylactic ... [https://www.sciencedirect.com/science/article/abs/pii/S221428942200148X]
- 21. Development of crosslinkable poly(lactic acid-co-glycidyl ... [https://www.nature.com/articles/pj2012159]
- 22. Fabrication of Crosslinked Poly(L-lactic acid) with ... [https://www.mdpi.com/2073-4360/17/22/3041]
- 23. Comparative Analysis of Crosslinking Methods and Their ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11051402/]
- 24. Star polylactide and its stereocomplex blends with ... [https://www.nature.com/articles/s41598-025-01961-9]
- 25. The Effect of Stereocomplexation and Crystallinity on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10934930/]
- 26. Stereocomplexation of Poly(lactic acid) and Chemical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7599924/]
- 27. Cross-linked structures reinforced the bamboo fiber/poly ... [https://www.frontiersin.org/journals/materials/articles/10.3389/fmats.2024.1484677/full]
- 28. In-situ cross-linking strategy for achieving excellent impact ... [https://pubmed.ncbi.nlm.nih.gov/39909258/]
- 29. Study on toughening polylactic acid/poly(butylene adipate ... [https://www.sciencedirect.com/science/article/abs/pii/S0141813025079279]
- 30. Polylactic Acid Blends Modified via In Situ Maleic ... [https://www.mdpi.com/2073-4360/17/16/2264]
- 31. Crystallization and Alkaline Degradation Behaviors of Poly ... [https://www.mdpi.com/2073-4360/12/10/2195]
- 32. Reactive compatibilization of PE/PS blends. Effect ... [https://www.sciencedirect.com/science/article/abs/pii/S0032386106013930]
- 33. Reactions at polymer–polymer interfaces for blend ... [https://www.sciencedirect.com/science/article/abs/pii/S0079670005000754]
- 34. Effects of Reactive Compatibilization on the Morphological ... [https://pubmed.ncbi.nlm.nih.gov/20353236/]
- 35. Crystallinity effect on electron-induced molecular structure ... [https://www.sciencedirect.com/science/article/abs/pii/S0032386122010977]
- 36. Influence of Infill Geometry and Density on the Mechanical Properties of 3D-Printed Polylactic Acid Structure | MDPI [https://www.mdpi.com/2504-4494/9/4/134]
- 37. Two-Stage Hybrid Optimization of Topology and Infill Density in Polymer Extrusion Additive Manufacturing for Lightweight High-Integrity Structures [https://www.mdpi.com/2076-3417/15/22/12258]
- 38. Enhancing Mechanical Properties of 3D-Printed PLA ... [https://www.mdpi.com/2504-477X/9/4/180]
- 39. Data-driven prediction of mechanical properties of 3D ... [https://www.sciencedirect.com/science/article/pii/S1359835X2500377X]
- 40. Development and Applications of PLGA Hydrogels for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11353330/]
- 41. A Comprehensive Review of Thermosensitive Hydrogels [https://pmc.ncbi.nlm.nih.gov/articles/PMC12294456/]
- 42. A Comparative Review on Biodegradation of Poly(Lactic ... [https://www.mdpi.com/2673-9623/5/1/1]
- 43. Review on the Degradation of Poly(lactic acid) during Melt ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10181416/]
- 44. A Comprehensive Review of Thermosensitive Hydrogels [https://www.mdpi.com/2310-2861/11/7/544]
- 45. Extremely stretchable thermosensitive hydrogels by ... [https://www.nature.com/articles/ncomms6124]
- 46. A review of biomaterial degradation assessment ... [https://www.nature.com/articles/s41529-024-00487-1]
- 47. Advanced Biocompatible and Biodegradable Polymers [https://pmc.ncbi.nlm.nih.gov/articles/PMC12611034/]
- 48. Medical applications and prospects of polylactic acid ... [https://www.sciencedirect.com/science/article/pii/S2589004224027391]
- 49. Effect of reinforcing particles on hydrolytic degradation ... [https://www.sciencedirect.com/science/article/abs/pii/S1359836816302700]
- 50. Recent advances in sustainable natural fiber composites [https://www.sciencedirect.com/science/article/pii/S2589234725001496]
- 51. Recent advances in biodegradable polymer blends and their ... [https://pubs.rsc.org/en/content/articlehtml/2025/gc/d5gc01294e]
- 52. Recent Approaches to the Plasticization of Poly(lactic Acid) ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10781029/]
- 53. Critical Review on Polylactic Acid: Properties, Structure ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9228835/]
- 54. Biodegradable Polylactic Acid and Its Composites [https://www.mdpi.com/2073-4360/15/14/3096]
- 55. Reactive plasticization and reprocessing effects [https://www.sciencedirect.com/science/article/abs/pii/S0141391018300168]
- 56. Crosslinking-induced compatibility and toughness ... [https://www.sciencedirect.com/science/article/abs/pii/S0141813025002387]
- 57. Effect of cross-linking on the microstructure and ... [https://pubmed.ncbi.nlm.nih.gov/16239799/]
- 58. Changes in Crystal Structure and Accelerated Hydrolytic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8707235/]
- 59. Toxic effects of biodegradable polylactic acid nanoplastics ... [https://www.nature.com/articles/s41598-025-21849-y]
- 60. Morphologies, Compatibilization and Properties of Immiscible PLA-Based Blends with Engineering Polymers: An Overview of Recent Works - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11244106/]
- 61. Boosting PLA melt strength by controlling the chirality of co-monomer incorporation - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8179584/]
- 62. Improving the performance of polylactic acid/polypropylene ... [https://www.sciencedirect.com/science/article/abs/pii/S0141813024086240]
- 63. Promoting crystallization of polylactide by the formation ... [https://www.sciencedirect.com/science/article/abs/pii/S0167577X13016571]
- 64. Biomimetic layered, ecological, advanced, multi-functional ... [https://www.nature.com/articles/s41467-025-61693-2]
- 65. PLGA Implants for Controlled Drug Delivery and ... [https://www.mdpi.com/1424-8247/18/5/631]
- 66. Application of PLGA in Tumor Immunotherapy - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11085488/]
- 67. End group chemistry modulates physical properties and ... [https://pubs.rsc.org/en/content/articlehtml/2025/tb/d5tb00816f]
- 68. PEGylated PLGA nanoparticles: unlocking advanced ... [https://molecular-cancer.biomedcentral.com/articles/10.1186/s12943-025-02410-x]
- 69. Multifunctional PLGA/collagen/zeolitic imidazolate ... [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1611948/full]
- 70. Advances in PCL, PLA, and PLGA-Based Technologies for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12566892/]
- 71. Enhancing the degradability of poly(lactic acid) through ... [https://www.nature.com/articles/s41428-025-01106-9]
- 72. A model-based protocol to quantify scission-crosslinking ... [https://www.sciencedirect.com/science/article/abs/pii/S1385894725015657]
- 73. Improving film-forming properties of PLA via nanocrystal ... [https://www.sciencedirect.com/science/article/abs/pii/S0032386125012534]
- 74. Impact of Degradation and Material Crystallinity on the ... [https://link.springer.com/article/10.1007/s10659-021-09835-7]
- 75. A multi-scale method for modeling degradation of ... [https://www.sciencedirect.com/science/article/abs/pii/S1742706116307152]
- 76. In Silico Evaluation of In Vivo Degradation Kinetics of Poly ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11122488/]
- 77. Drug Release Kinetics and Transport Mechanisms of Non- ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2846103/]
- 78. Drug Carriers: A Review on the Most Used Mathematical ... [https://www.mdpi.com/2227-9717/10/6/1094]
- 79. An investigation of the drug release kinetics of 3D-Printed ... [https://www.sciencedirect.com/science/article/pii/S0378517325000547]
- 80. Applications of PLA in modern medicine - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7474829/]
- 81. Enhancing Polylactic Acid (PLA) Performance: A Review of ... [https://www.mdpi.com/2073-4360/17/2/191]
- 82. Mechanical and Geometric Performance of PLA-Based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7215744/]
- 83. Effect of Degradation During Multiple Primary Mechanical ... [https://www.mdpi.com/2073-4360/17/18/2542]
- 84. Properties and Biodegradation of Poly(lactic Acid) ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12114976/]
- 85. Achieving the simultaneous improvement of degradation ... [https://www.sciencedirect.com/science/article/pii/S1359836825003439]
- 86. Inherently degradable cross-linked polyesters and ... [https://pubs.rsc.org/en/content/articlehtml/2020/py/d0py01226b]
- 87. Changes in Heat Resistance and Mechanical Properties of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11859537/]
- 88. Properties and hydrolysis of PLGA and PLLA cross-linked ... [https://www.sciencedirect.com/science/article/abs/pii/S0141391010000662]
- 89. Longâ€Lasting Crossâ€Linked PLGAâ€Inspired Nanoparticles from Oneâ€Pot Nanopolymerization of Precisely Sequenced Short Oligolactoglycolic Acid Dimethacrylates - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12004898/]
- 90. Impact of terminal groups on PLGA degradation rate and ... [https://pubmed.ncbi.nlm.nih.gov/41238007/]
- 91. Drug-encapsulated blend of PLGA-PEG microspheres: in vitro and in vivo study of the effects of localized/targeted drug delivery on the treatment of triple-negative breast cancer | Scientific Reports [https://www.nature.com/articles/s41598-020-71129-0]
- 92. Effectiveness of drug-loaded polyethylene glycol-poly lactic ... [https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2025.1710176/full]
- 93. PEGylated PLGA nanoparticles: unlocking advanced ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12288292/]
- 94. Design of Poly(lactic-co-glycolic acid) nanoparticles in drug ... [https://www.sciencedirect.com/science/article/abs/pii/S0169743925000206]
- 95. The Life Cycle Assessment for Polylactic Acid (PLA) to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8199738/]
- 96. Polylactic Acid Market Size and Forecast 2025 to 2034 [https://www.precedenceresearch.com/polylactic-acid-market]
- 97. Thermal and mechanical properties of chemical ... [https://www.sciencedirect.com/science/article/abs/pii/S0142941808001372]
- 98. Cradle-to-grave life cycle assessment of single-use cups ... [https://www.sciencedirect.com/science/article/pii/S0921344921001154]
- 99. Impact of High-Dose Gamma Irradiation on PLA/PBAT ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12391937/]